
Immunohistochemical findings in small cell neuroendocrine carcinoma of the cervix: a report of two cases
Introduction
Small cell cancers most commonly arise in the lung; however, they can also occur at other uncommon sites, including the uterine cervix, esophagus, stomach, liver, pancreas, bladder, duodenum, prostate, breast, and skin (1,2). Small cell neuroendocrine carcinoma of the cervix (SCCC) is an uncommon gynecologic malignancy, accounting for 1–5% of all gynecologic malignancies (3). SCCC is extremely aggressive and has a poor prognosis because of the development of lymph node metastases and invasion, even in patients diagnosed at an early stage (2). However, because of the rarity of SCCC, a consensus diagnostic criteria, treatment guidelines, and optimal treatment strategies have not been determined thus far. To improve our understanding of SCCC and identify relevant factors, we carried out a retrospective case study.
Case presentation
Case 1
A 74-year-old woman, gravida 3, para 2 was admitted with postmenopausal vaginal bleeding. The patient had a 3-month history of spontaneous vaginal secretions. A cauliflower-like exophytic mass with a diameter of 3.5 cm and involvement of the vaginal posterior fornix regions was identified in the uterine cervix upon gynecological examination. The tissue was friable with hemorrhage. Transrectal ultrasonography revealed atypical hyperplasia of the uterosacral ligament, and that the compartment between the uterine tissue and the pelvic wall had disappeared; however, computed tomography (CT) imaging showed no abnormal density in the fallopian tubes, ovary, or omentum.
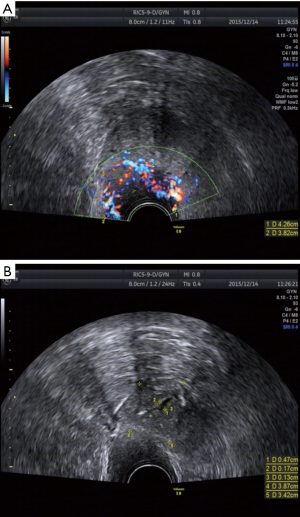
Vaginal ultrasound and CT scans were performed. On ultrasound examination, the uterine cervix appeared as a solid mass with a hypoechoic echo structure compared with the surrounding cervical stroma. There were small solid clumps in the cervix (dimension 43 mm × 38 mm) and hypoechoic masses with blurred boundaries. Transvaginal ultrasound imaging showed cervical prominence that was richly vascularized on power Doppler (Figure 1). A CT scan demonstrated a soft tissue nodule with inner heterogeneity measuring 35 mm × 29 mm in the left lower cervix; the margins were unclear.
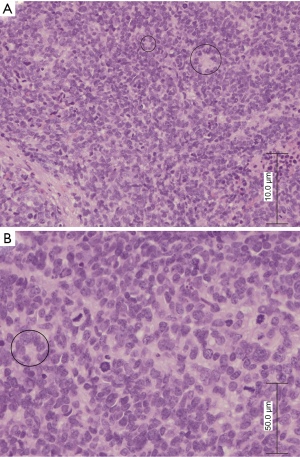
Tissue specimens were obtained using cervical biopsy. For paraffin-embedded tissue specimens, consecutive 3–5 µm thick sections were cut and used. Haematoxylin and eosin (H&E) staining revealed that small round tumor cells were arranged in sheets with rosette formation. The tumor cells had scant and absent cytoplasm, granular chromatin, indistinct nucleoli, nuclear atypia, and ill-defined cell borders. Individual cell necrosis with karyorrhexis and karyolysis were visible, but not especially prominent (Figure 2).
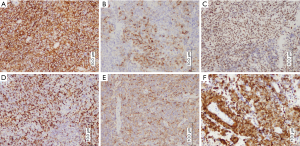
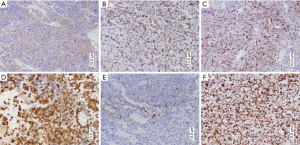
Immunohistochemical tests were performed for the tumor markers shown in Table 1. Immune positivity for CD56 was observed on cell membranes, for FLi-1 and P16 in the nucleus, and for epithelial membrane antigen (EMA), Vimentin, and Chromogranin A (CgA) in the cytoplasm (Figure 3). Immunohistochemical staining for CK was negative; the remaining staining results are shown in Table 2. High-risk human papilloma virus (HPV) was detected using Hybrid Capture 2. The extent of HPV infection (i.e., viral load) was 1,468.61 (RLU/CO).
Full table
Full table
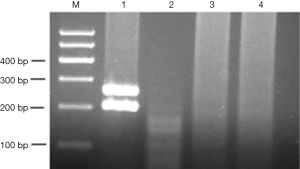
The EWS-FLI1 fusion gene was detected using One-Step real-time polymerase chain reaction (RT-PCR). The t(Jeny11;Jeny22)(q24;q12) has been consistently detected involving chromosomal breaks in FLI1 intron 5 was 277 bp. The primers used were 5'-ACTCCCCGTTGGTCCCCTCC-3'. Thermal cycling was performed with reverse denaturation at 50 °C for 30 min and initial denaturation at 95 °C for 15 min. Forty cycles for denaturation at 94 °C for 30s, annealing at 58 °C for 45 s, and extension at 72 °C for 1 min were performed, followed by total extension at 72 °C for 10 min. This reagent purchased from QIAGEN Company. Our patient tested negative for EWS-FLI1 fusion gene expression. We used the primary primitive neuroectodermal tumor (PNET) as a positive control and synovial sarcoma as a negative control (Figure 4). The diagnosis of SCCC is based on accurate histopathological and immunohistochemical identification of neuroendocrine features. We eliminated lymphoma and PNET, and neuroendocrine SCCC was diagnosed On September 30, 2015.
The patient subsequently underwent whole pelvis radiation of 40 Gy in 20 fractions, and chemotherapeutic agents, including cisplatin and etoposide, were used in therapy. However, after 2 cycles of treatment, toxicity reactions of radiocystitis and radiation proctitis developed. The patient developed pulmonary metastasis; CT demonstrated a soft tissue nodule with inner heterogeneity measuring 24 mm × 27 mm in the right lung. The patient died of lung metastasis from the progressive disease after 5 months.
Case 2
A 45-year-old woman, gravida 4, para 2 presented to her primary physician with complaints of abnormal bleeding after intercourse for 6 months. The patient’s condition gradually worsened; however, she had not sought medical advice until 2 months previously when these symptoms had become increasingly severe.
The patient was examined using cervical biopsy. The size and shape of the cervix distorted by the presence of a large neoplasm located on the anterior wall of the cervix. Vaginal ultrasound performed and revealed a low-echogenic mass.
Microscopically, the specimens showed small round cells arranged in sheets with palisading. The tumor cells were round or ovoid, and were a consistent size with scanty amounts of cytoplasm, inconspicuous nucleoli, and hyperchromatic nuclei (Figure 5).

The immunohistochemical details of the cases are summarized in Table 1. Strong cytoplasmic staining for CgA and Vimentin were observed. Scattered neoplastic cells were positive for Syn. The majority of malignant cells demonstrated intensely positive cytoplasmic staining for Neuro-specific Enolase (NSE). CD56 showed positive immunoreactivity in the cell membrane, while the cell nucleus was intensely positive for p16 and Ki-67 (Figure 6). The tumor cells were negative for CK and LCA. The remaining staining results are provided in Table 2.
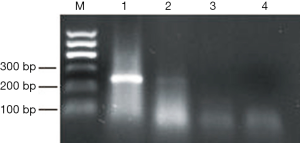
Expression of EWS-FLI1 fusion gene was detected using the One-Step RT-PCR kit (Qiagen). EWS-FLI1 fusion gene expression in our patient was negative (Figure 7). The final diagnosis was stage IIA2 SCCC on December 28, 2015. The patient underwent laparoscopic radical hysterectomy, bilateral salpingectomy, and pelvic lymphadenectomy; postoperative adjuvant chemotherapy and radiotherapy were not performed. However, she died 7 months after the surgery.
Discussion
SCCC of the female genital tract is a subtype of neuroendocrine tumors (NETs); the incidence of NET, particularly SCCC, is very rare, accounting for 1–2% (range, 0.5–5%) of all cervical malignancies (4). SCCC was first described by Wentz and Reagan in 1958 (5) and is a highly malignant disease. Gardner et al. (6) reported that the patient age ranges from 21 to 87 years and the median age is 50 years. The presenting symptom is most commonly vaginal bleeding. The precursor lesion is often nonspecific, and the clinical manifestations and predilection site of SCCC are similar to those in cervical cancer patients. SCCC is difficult to distinguish from other small round-cell tumors, such as lymphoma, primary PNET, and poorly differentiated carcinoma, based on the morphological criteria. Diagnosis of SCCC requires tissue biopsy for routine H&E staining, immunohistochemistry for neuroendocrine markers, and systemic imaging of the chest, abdomen, and pelvis to rule out the possibility that this represents a metastasis from another site. SCCC exhibits various degrees of neuroendocrine differentiation, as detected using conventional morphological, ultrastructural, histochemical, and/or immunohistochemical criteria. These tumors are characterized by a high incidence of lymphatic and vascular invasion, as well as distant organ metastases, in patients with early-stage disease, resulting in a poorer prognosis. Abeler et al. (4) reported that the 5-year survival rate of patients with SCCC was 14%. The majority of patients presented with irregular vaginal bleeding, discharge, post-coital spotting, and pelvic pain or pressure.
Because of the non-specific features of SCCC, the morphological criteria of small cell neuroendocrine carcinoma were determined when H&E staining of the tumor revealed most of the following features. At a low magnification, the tumor cell distribution was island-like, sheet-like, or palisading-like. At a higher magnification, the tumor generally showed uniform small round, oval or fusiform cells of small size, with scant or absent amounts of cytoplasm, granular chromatin, significant nuclear atypia, inconspicuous or prominent nucleoli, hyperchromatic nuclei, multifocal necrosis, and ill-defined or well-circumscribed cell borders.
In our study, we found that tumor cells were arranged in sheets with palisading. The tumor cells were round or ovoid, and of a consistent size with scant and absent cytoplasm, granular chromatin, indistinct nucleoli, and nuclear atypia. We suggest that immunocytochemical and EWS-FLI1 fusion transcript analysis should be performed for the differential diagnosis of SCCC.
A study has indicated that, typically, immunohistochemistry for 3 markers—CD56, synaptophysin, and chromogranin—should be performed for confirmation of SCCC. Albores-Saavedra et al. (7) confirmed that not all of the markers need to be positive to make the diagnosis because 60% of cases are negative for chromogranin-A and synaptophysin, and 30% are negative for NSE. We found that the tumor cells were positive for CD56, Syn, CgA, NSE, Vimetin, FLi-1, and CD99; these markers are helpful in confirming the diagnosis in cases that are difficult to identify. CD56 is a marker of NK cell lymphomas, and is often used as a marker of lung NET. Albores-Saavedra et al. (7) found that CD56 was the most sensitive marker, and in their series the positive rate was 88% (22/25). Syn has been reported as the most specific and sensitive marker of neuroendocrine differentiation. CgA is widely expressed in NET cells and tumor cells, and is mainly used as a marker of NET. CD99 and FLi-1 are mainly used in the diagnosis of primary PNET. In our cases, CD99 and FLi-1 were negative in the case 2; however, in case 1, FLi-1 was positive. LCA is commonly used as a marker to distinguish cells of the lymphoid lineage from those of the myeloid lineage. Therefore, we eliminated lymphoma from our differential diagnosis.
Microscopically, primary PNET appears as a small blue round cell tumor. H&E staining reveals small round tumor cells with absent cytoplasm, granular chromatin, significant nuclear atypia, prominent nucleoli, and tumor cells with an organoid arrangement, with trabecular, insular, and spindle patterns of growth. Primary PNET can be difficult to identify using cell morphology. The clinical history of cancer and immunohistochemistry is helpful in the differential diagnosis of primary PNET and SCCC.
In SCCC, most of the neuroendocrine-related immunohistochemical markers were positive. However, in primary PNET, neuroendocrine-related immunohistochemical markers were generally not expressed. In our cases, EWS-FLI1 fusion transcripts were detected in the specimens. Primary PNET was ruled out based on expression findings. One-step RT-PCR is a useful assay to detect the expression of the specific translocation fusion transcripts in soft tissue sarcomas. The expressions of EWS-FLI1 and other fusion transcripts are reliable molecular markers, and are helpful in establishing the diagnosis and differential diagnosis of a primary PNET, Ewing’s sarcoma. The chromosomal translocation of t (Jeny11;Jeny22) (q24;q12) is present in the majority of cases of PNET and Ewing’s sarcoma (8,9).
Mechanisms of SCCC tumorigenicity have not been fully elucidated. HPV is an oncogenic virus. Persistent infections with HPV are necessary for invasive cervical cancer or vulvar cancer, which is commonly associated with HPV genital infection and typically occurs in younger women (10). Epidemiologic analysis showed that the most cervical cancer patients were positive for HPV infection. Stoler et al. (11) showed that 14 of 18 cases showed HPV with neuroendocrine differentiation. Lindel et al. (12) reported that 76.2% (64/84) of patients with SCCC had been infected with HPV. Our patients had also been infected with HPV. In one of the patients, the viral load was 1,468.61 (RLU/CO). When the viral load is >100, significantly increased the positive rate of cervical cancer lesions before (P<0.005), and when the HPV DNA viral load >500, the incidence of cervical cancer was significantly increased (P<0.001). Several studies have shown HPV types are the most important risk factors for the development of cancers, especially the high-risk HPV types 16 and 18. Some reports have indicated that mechanisms of tumorigenicity are modulated factors, such as peroxisome proliferator-activated receptors (PPARs) and nuclear factor-кB (NF-кB); Laganà et al. (13) reported that PPARs have an important effect on the risk of cancer. More studies are needed into the pathogenesis of SCCC to better provide information and support for researchers and clinicians.
Given the rarity of SCCC, data are limited on the optimal treatments for SCCC; the vast majority of research investigating treatment is retrospective and based on relatively some case reports and a small series. Appropriate treatments for cervical cancer depend on the stage of the cancer, the size of the tumor, the desire to preserve fertility, and patient age. Neuroendocrine small cell carcinoma is reported to cause distant metastases at an early stage and adjuvant chemotherapy may be necessary. In this report, one of the patients was treated with a combined radiation treatment with chemotherapeutic agents, such as cisplatin and etoposide, for 4 weeks. The other patient underwent surgery. Pazdur et al. (14) first suggested a chemotherapeutic regimen for SCCC based on their experience of the treatment of small cell carcinoma of the lung (SCLC); the biologic and morphological behavior of SCCC resembles that of SCLC. Someone showed that etoposide-based chemotherapy might prevent distant organ metastasis in patients with early-stage disease of SCCC. In a single-case study, it was reported that long-term survival was affected by a chemotherapeutic combination of etoposide with platinum in a patient with early-stage disease. Early stage (Stage I–IIA) neuroendocrine cervical carcinoma is typically treated with radical hysterectomy, pelvic lymphadenectomy (LAD), and adjuvant chemotherapy. Radical hysterectomy followed by adjuvant chemotherapy resulted in a higher 2-year survival rate compared with radical hysterectomy followed by adjuvant radiotherapy (62.5% vs. 16.7%). However, for some younger patients suffering from SCCC, a conservative therapeutic approach is best to preserve their fertility. Singh et al. (15) described a 26-year-old Gravida 2 Para 2 patient who was diagnosed with Stage IB1 small cell neuroendocrine carcinoma of the cervix and was successfully treated with fertility-sparing surgery (radical trachelectomy and bilateral pelvic LAD) and subsequent adjuvant multi-agent chemotherapy. A 27-year-old nulliparous Caucasian woman with early stage large cell neuroendocrine carcinoma (LCNEC) who underwent radical trachelectomy was the first patient to successfully conceive and deliver a child after fertility-sparing treatment, following radical trachelectomy for LCNEC (16). Except SCCC, Rossetti et al. (17) have been reported when early stage endometrial cancer is diagnosed in young woman, a conservative approach should be considered; the patients conceived at the first in vitro fertilization attempt in their study.
Several studies have found that disease stage is the most accurate prognostic factor, although other factors (age, race, tumor size, depth of stromal invasion, vascular space invasion, and emotional state) are prognostic factors in SCCC. Research has shown that women suffering from extremely aggressive gynecological cancers, including SCCC, endometrial cancer, vulvar cancer, and ovarian cancer, experience negative consequences in terms of sexual function, quality of life, and mental health (18,19). The relevant doctors should be able to provide adequate counseling and support to reduce the risk of adverse effects on the sexual activity and psychological functioning of patients and their partners (20). It is important to note that most of the studies conducted in this field focus on the medical aspects. Psychological and social factors should also be investigated to provide patient with optimal care.
In summary, SCCC is rare and highly aggressive. Early diagnosis is especially necessary because of its dismal prognosis, early metastasis, and high mortality. Combination surgery, chemotherapy, and radiotherapy treatment are usually used to treat SCCC, although evidence of their efficacy remains inconclusive. Despite these discoveries, studies delved into the role of SCCC diagnosis, therapy and pathogenesis have yet been unidentified. In order to investigate SCCC, we have reported 2 rare cases of SCCC. Our report will contribute to the understanding of SCCC.
Acknowledgments
The authors are grateful to the Key Laboratory of Xinjiang Endemic and Ethnic Diseases (Ministry of Education), Shihezi University School of Medicine, for assistance with this work.
Funding: Financial & competing interests disclosure Support was provided by the National Natural Science Foundation of China (No. 81560053), the Corps Doctor Foundation (No. 2014BB018), One Thousand Youth Talents Plan, the Pairing Program of Shihezi University with Eminent Scholar in Elite University (No. SDJDZ201508).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.07.15). The authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the next of kin of the patients for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Crowder S, Tuller E. Small cell carcinoma of the female genital tract. Semin Oncol 2007;34:57-63. [Crossref] [PubMed]
- Viswanathan AN, Deavers MT, Jhingran A, et al. Small cell neuroendocrine carcinoma of the cervix: outcome and patterns of recurrence. Gynecol Oncol 2004;93:27-33. [Crossref] [PubMed]
- Scully RE, Aguirre P, DeLellis RA. Argyrophilia, serotonin, and peptide hormones in the female genital tract and its tumors. Int J Gynecol Pathol 1984;3:51-70. [Crossref] [PubMed]
- Abeler VM, Holm R, Nesland JM, et al. Small cell carcinoma of the cervix. A clinicopathologic study of 26 patients. Cancer 1994;73:672-7. [Crossref] [PubMed]
- Wentz WB, Reagan JW. Survival in cervical cancer with respect to cell type. Cancer 1959;12:384-8. [Crossref] [PubMed]
- Gardner GJ, Reidy-Lagunes D, Gehrig PA. Neuroendocrine tumors of the gynecologic tract: A Society of Gynecologic Oncology (SGO) clinical document. Gynecol Oncol 2011;122:190-8. [Crossref] [PubMed]
- Albores-Saavedra J, Gersell D, Gilks CB, et al. Terminology of endocrine tumors of the uterine cervix: results of a workshop sponsored by the College of American Pathologists and the National Cancer Institute. Arch Pathol Lab Med 1997;121:34-9. [PubMed]
- Ladanyi M. Translocation-based molecular diagnosis of sarcomas. Am J Surg Pathol 2003;27:414-5; author reply 5-6. [Crossref] [PubMed]
- Hill DA, O'Sullivan MJ, Zhu X, et al. Practical application of molecular genetic testing as an aid to the surgical pathologic diagnosis of sarcomas: a prospective study. Am J Surg Pathol 2002;26:965-77. [Crossref] [PubMed]
- Vitale SG, Valenti G, Biondi A, et al. Recent trends in surgical and reconstructive management of vulvar cancer: review of literature. Updates Surg 2015;67:367-71. [Crossref] [PubMed]
- Stoler MH, Mills SE, Gersell DJ, et al. Small-cell neuroendocrine carcinoma of the cervix. A human papillomavirus type 18-associated cancer. Am J Surg Pathol 1991;15:28-32. [Crossref] [PubMed]
- Lindel K, Burri P, Studer HU, et al. Human papillomavirus status in advanced cervical cancer: predictive and prognostic significance for curative radiation treatment. Int J Gynecol Cancer 2005;15:278-84. [Crossref] [PubMed]
- Laganà AS, Vitale SG, Nigro A, et al. Pleiotropic Actions of Peroxisome Proliferator-Activated Receptors (PPARs) in Dysregulated Metabolic Homeostasis, Inflammation and Cancer: Current Evidence and Future Perspectives. Int J Mol Sci 2016;17:E999 [Crossref] [PubMed]
- Pazdur R, Bonomi P, Slayton R, et al. Neuroendocrine carcinoma of the cervix: implications for staging and therapy. Gynecol Oncol 1981;12:120-8. [Crossref] [PubMed]
- Singh S, Redline R, Resnick KE. Fertility-sparing management of a stage IB1 small cell neuroendocrine cervical carcinoma with radical abdominal trachelectomy and adjuvant chemotherapy. Gynecol Oncol Rep 2015;13:5-7. [Crossref] [PubMed]
- Rajkumar S, Iyer R, Culora G, et al. Fertility sparing management of large cell neuroendocrine tumour of cervix: A case report & review of literature. Gynecol Oncol Rep 2016;18:15-7. [Crossref] [PubMed]
- Rossetti D, Bogani G, Carnelli M, et al. Efficacy of IVF following conservative management of endometrial cancer. Gynecol Endocrinol 2014;30:280-1. [Crossref] [PubMed]
- Laganà AS, La Rosa VL, Rapisarda AM, et al. Comment on: "Needs and priorities of women with endometrial and cervical cancer J Psychosom Obstet Gynaecol 2017;38:85-86. [Crossref] [PubMed]
- Vitale SG, La Rosa VL, Rapisarda AM, et al. Comment on: "Anxiety and depression in patients with advanced ovarian cancer: a prospective study J Psychosom Obstet Gynaecol 2017;38:83-4. [Crossref] [PubMed]
- Vitale SG, La Rosa VL, Rapisarda AM, et al. Comment on: "The consequences of gynaecological cancer in patients and their partners from the sexual and psychological perspective Prz Menopauzalny 2016;15:186-7. [Crossref] [PubMed]


