
Metabolic volume parameters based on different thresholds with baseline 18F-FDG PET/CT as prognostic factors for survival in stage III non-small cell lung cancer
Introduction
Stage III non-small-cell lung cancer (NSCLC), comprising 25% to 30% of the total cases of NSCLC, manifests in a range of heterogeneous patients, from those with potentially operable lesions to those with inoperable advanced lesions (1,2). There is a marked inhomogeneity in outcomes in patients with stage III NSCLC presenting with the same performance status (3,4). At present, concurrent chemo-radiotherapy is recommended as the standard treatment for nonsurgical stage III NSCLC patients. Disappointingly, the 5-year survival has not significantly improved during the past decade (5,6). To improve the outcomes of patients, better and more detailed individualized treatments must be developed, and doing so depends on the identification of a more reliable prognostic factor. Therefore, it is essential to find prognostic factors for specific cohort of patients.
Fluorine 18 fluorodeoxyglucose (FDG) positron emission tomography (PET) is widely used for diagnosis and staging in lung cancer, and volume-based metabolic parameters, such as the metabolic tumor volume (MTV) and total lesion glycolysis (TLG), have been investigated for their prognostic value in NSCLC. However, there is still no consensus in the literature on the prognostic value of metabolic parameters (7-11).
Malignant tumors are notably heterogeneous and composed of different cell subgroups with different characteristics of perfusion, hypoxia, cell density, and proliferation (12-14). Tumor recurrence or metastasis depends primarily on these specific cell subgroups (15-18). Studies have indicated that the intratumoral heterogeneity can be described by the variability in the voxel intensity of FDG PET within the tumor volume (TV) (19,20). Consequently, we predicted a ladder-like distribution of the tumor metabolism on which different levels may represent diverse biological characteristics. Hence, we presumed that different thresholds of the MTV or TLG might serve as prognostic factors.
Our study focused on the prognostic value of the MTV with different SUV thresholds of FDG PET/CT. Our intention was to refine the prognostic analysis of the volume-based metabolic parameters of FDG PET/CT in patients with nonsurgical stage III NSCLC.
Methods
Patients
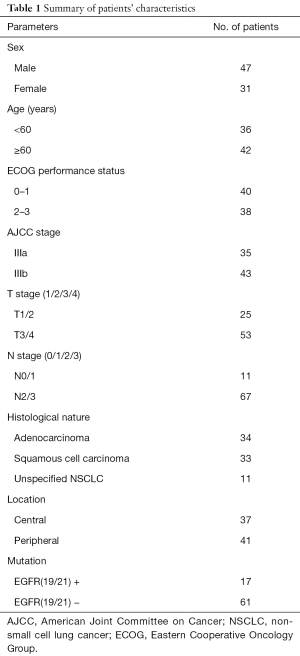
A total of 78 cases of stage III NSCLC (AJCC stage) were confirmed by histology or cytology and treated with concurrent chemo-radiotherapy in the Radiotherapy Department in Shandong Cancer Hospital between January 2009 and December 2012. All cases had undergone FDG PET/CT pretreatment and subsequently concurrent chemo-radiotherapy. We conducted the study under the review and approval of the Institutional Review Committee of the Cancer Hospital of Shandong Province, and all the patients signed informed consent (ID of Ethics Approval: 201208020). The median interval from PET/CT to chemo-radiotherapy was 11 days (range 4–21 days). Before treatment, the patients were examined for staging, which included bronchoscopy, transthoracic biopsy, CT or MR of the brain, and contrast-enhanced CT of the chest and abdomen. The exclusion criteria were previous thoracic surgery, chemotherapy or radiotherapy or diabetes. We recorded the clinical variables, such as sex, age, stage, histology, ECOG performance status, EGFR mutations, tumor location. NSCLC could be divided into central and peripheral tumors by its location. Central tumors were defined as those within 2 cm of the proximal bronchial tree or immediately adjacent to mediastinal/pericardial pleura; Tumors that did not meet this criterion were defined as peripheral-type tumors (21). The patients’ characteristics are summarized in Table 1.
Full table
After the completion of the treatment, all patients were followed up every three months. A physical examination and contrast-enhanced CT of the chest and abdomen were included in the follow-up evaluation. All the patients were followed up until December 31st 2015 or their date of death, and the median follow-up time was 24.5 months (range 12–39 months). Each of patients was scored for the endpoints of overall survival (OS) and progression-free survival (PFS). OS was defined as the time from the day of the treatment beginning to the day of the last follow-up or death, and PFS was calculated from the day of the treatment beginning to the day of local or distant disease progression. The estimated median PFS was 11.0 months (range 3–23 months). The estimated median OS was 19.0 months (range 10–38 months).
18F-FDG PET/CT imaging/analysis
All patients rested and fasted for more than 6 h before the PET/CT (Discovery LS PET/CT system, GE Healthcare) examination. Briefly, the serum glucose was determined to ensure controlled levels below 6.6 mmol/L. Patients waited 55±5 min (range, 45–60 min; median, 55 min) after the intravenous infusion of 10 mCi of 18F-FDG, and then PET imaging was performed from the head to the upper thighs. Both the PET and the CT images were taken during normal breathing. The PET images were reconstructed by using the ordered subset expectation maximization (OSEM) algorithm with the CT-derived attenuation correction. The PET images, attenuation-corrected CT images, and fusion PET/CT images were displayed as coronal, axial and sagittal planes. We viewed and analyzed these images on a Xeleris workstation (GE Healthcare).
The parameters of the PET/CT images, which included the MTV, TLG, SUVmean, and SUVmax of the primary tumor, were assessed and recorded by two experienced nuclear medicine physicians. MTV represents the TV with an SUV equal to or greater than a specified threshold. We computed MTV2.5 on the basis of an absolute SUV threshold of 2.5. We also evaluated MTV values on the basis of relative thresholds, i.e., MTV40%, MTV50%, MTV60%, MTV70%, MTV80%, and MTV90%, which were defined as volumes with an SUV greater than 40%, 50%, 60%, 70%, 80%, and 90% of SUVmax, respectively. The MTV was automatically delineated on the PET/CT images with the specified SUV, such as a contour line, and then calculated with the software. The SUVmax and SUVmean for each MTV were also calculated automatically. TLG50% was calculated as MTV50% multiplied by the SUVmean, and TLG60% was calculated in a similar manner. To obtain the CT based tumor volume (CTV), we also delineated the primary tumor and calculated the volume on CT scan, using the lung window setting (window width, 1,000 HU; window center, −700 HU) as our previous research did (22).
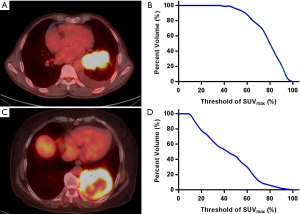
The area under the curve of cumulative SUV-volume histograms (AUC-CSH) was used to evaluate intratumoral metabolic heterogeneity, and the CSH was obtained by plotting the percent volume of a tumor with a SUV above a certain threshold against that threshold, which was varied from 0 to 100% of SUVmax (23). In order to calculate the percent volume of the tumor, the threshold SUV intensity value exceeding 3.0 was used as the region of interest (ROI) (24). AUC-CSH was extracted from the ROI. Image analysis was performed using an in-house MATLAB code (Mathworks Inc., Natick, USA). Figure 1 shows the typical examples of homogeneity (A) and heterogeneity (C) FDG uptake in two patients, whose AUC were 0.74 (B) and 0.37 (D) respectively.
Chemo-radiotherapy
All the patients underwent radiotherapy, which was performed with megavoltage equipment (6 MV) with an intensity-modulated radiotherapy (IMRT)/three-dimensional conformal radiotherapy (3D-CRT) technique. Thoracic radiotherapy was implemented as conventionally fractionated radiotherapy with 5× 2 Gy per week to a total dose of at least 60 Gy. The radiotherapy plan was based on a planning CT image. The gross tumor volume (GTV) comprised the primary tumor and included the lymph nodes, and the planning target volume (PTV) comprised the GTV with an edge expansion of 10–15 mm. All patients underwent two cycles of concurrent chemo-radiotherapy with cisplatin-based doublet chemotherapy, with or without adjuvant chemotherapy.
Statistical analysis
We analyzed the data by using SPSS version 22.0 (SPSS, Inc.; IBM Co.). The Cox proportional hazards model was used to estimate variables for the univariate and multivariate analyses in our study. These variables included the PET/CT parameters and some clinical variables, such as sex, age, tumor location, ECOG, stage and histology. For the intent of statistical analyses, clinical variables were divided into two or three categories; For the age of patients, 60 years was selected as the cutoff for classification routinely; SUVmax, AUC-CSH, CTV, MTV2.5, MTV40%, MTV50%, MTV60%, MTV70%, MTV80%, MTV90%, TLG50%, TLG60% were considered as binary variables by dividing patients into pairs of subgroups on the basis of the median values of the above parameters. The differences in the estimated factors among the subgroups were evaluated using the log-rank test.
Also, we evaluated correlation coefficients of the independent variables. We used Spearman rank correlation for non-continuous variables, and Pearson correlation for continuous variables. The correlation coefficients between the PET parameters and the CTVs (MTV2.5vs. CTV, MTV40%vs. CTV, MTV50%vs. CTV, TLG50%vs. CTV, TLG60%vs. CTV) were higher than 0.700 (0.985, 0.883, 0.802, 0.793, 0.755 respectively), and the correlation coefficients between the PET parameters (MTV2.5vs. MTV40%, MTV40%vs. MTV50%, MTV70%vs. MTV80%, MTV50%vs. TLG50%, TLG50%vs. TLG60%)were also very high (0.856, 0.922, 0.710, 0.781, 0.922, respectively), therefore, to avoid multicollinearity, each multiple variable regression model included one PET parameter or CTV and other clinical variables. Differences were considered to be significant when the two-tailed P value was less than 0.05.
Results
PET/CT parameters of primary tumor
In order to analyze the prognostic value of metabolic volume parameters, we assessed and recorded each patient’s PET/CT metrics. The median SUVmax of the primary tumor was 13.0 (range 3.8-22.0), and the median values of TLG50% and TLG60% were 111.5 (range 8.6–547.7) and 74.1 (range 5.4–479.7), respectively. The median MTV values in mL were MTV2.5 of 52.1, MTV40% of 29.6, MTV50% of 15.8, MTV60% of 8.7, MTV70% of 4.0, MTV80% of 1.5, and MTV90% of 0.3.
PET/CT parameters and survival
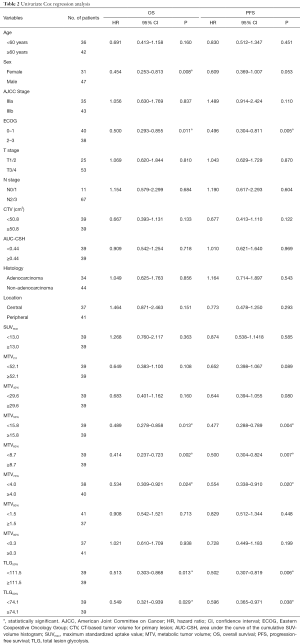
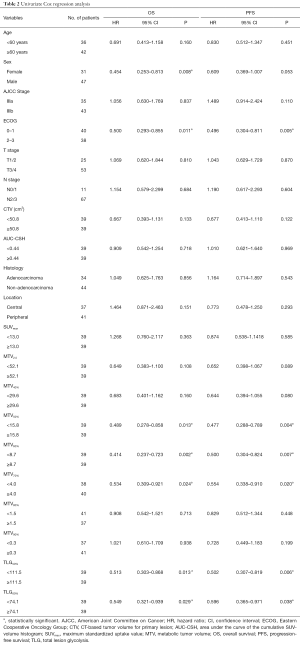
To evaluate the prognostic impacts of patients’ characteristics and the PET/CT parameters, we used the univariate analyses for OS and PFS with Cox Proportional Hazards Model. In the univariate analysis, MTV50%, MTV60%, MTV70%, TLG50%, TLG60% and sex, ECOG were significant prognostic factors of OS [HR =0.489, P(MTV50%)=0.013; HR =0.414, P(MTV60%)=0.002; HR =0.534, P(MTV70%)=0.024; HR=0.513, P(TLG50%)=0.013; HR =0.549, P(TLG60%)=0.029; HR=0.454, P(sex)=0.008, HR=0.500, P(ECOG)=0.011], while MTV50%, MTV60%, MTV70%, TLG50%, TLG60% and ECOG were significantly associated with PFS [HR =0.477, P(MTV50%)=0.004; HR=0.500, P(MTV60%)=0.007; HR=0.554, P(MTV70%)=0.020; HR =0.502, P(TLG50%)=0.006; HR =0.596, P(TLG60%)=0.038; HR =0.496, P(ECOG)=0.005]. Detailed results of this analysis are shown in Table 2. The HRs of variables, such as age ≥60 years, male, IIIb, ECOG 2–3, T3/T4, N2/N3, non-adenocarcinoma, and peripheral type were all 1.0.
Full table
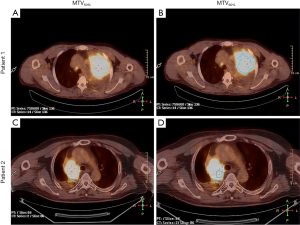
In the multivariate analysis, TLG50% was significantly associated with OS (HR =0.423, P=0.023), and also significant prognostic factor of PFS [HR =0.457, P(TLG50%)=0.029]. MTV60% was significantly associated with PFS (HR =0.402, P(MTV60%)=0.042). SUVmax was not an independent prognostic factor for nonsurgical stage III NSCLC in terms of the OS or PFS (HR =0.885, P=0.717; HR =0.928 P=0.809). These PET/CT parameters were separately analyzed and were adjusted for age, sex, stage, histology, ECOG, and location. Figure 2 shows a typical example of two patients with stage III NSCLC. They had similar values of MTV50% (34.6 vs. 37.4 mL) but different values of MTV60% (10.4 vs. 21.2 mL). The patient with a lower MTV60% had a longer PFS (13 vs. 7 months). Detailed results of this analysis are shown in Table 3.
Full table
Prognostic stratification by MTV60% and TLG50%
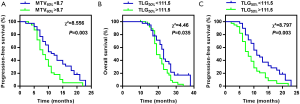
To validate the prognostic roles of MTV60% and TLG50%, we divided the patients into two pairs of subgroups on the basis of the median values of MTV60% and TLG50%. Figure 3A shows the PFS for patients with MTV60% above or below the median. The PFS difference between the groups was significant, with a P value of 0.003, and patients with a higher MTV60% demonstrated a worse survival. Patients with a MTV60% more than 8.7 mL demonstrated worse PFS (median 9 months; 95% CI: 6.986 to 11.014) compared to less than 8.7 mL (median 12 months; 95% CI: 9.990 to 14.010). Figure 3B,C show the OS and PFS for patients with TLG50% above or below the median respectively. Improved OS and PFS were significantly associated with a lower TLG50% (P=0.035, P=0.003). TLG50% more than 111.5 (median 19 months; 95% CI: 16.835 to 21.165) demonstrated worse OS compared to less than 111.5 (median 23 months; 95% CI: 21.820 to 24.180), and TLG50% more than 111.5 (median 9 months; 95% CI: 6.961 to 11.039) demonstrated worse PFS compared to less than 111.5 (median 12 months; 95% CI: 10.381 to 13.619).
Discussion
A prognostic assessment of pretreatment is important for improving survival because it enables patients to be stratified into optimal therapeutic regimens. We analyzed the prognostic roles of SUVmax and different thresholds of MTV and TLG in addition to the clinical variables of nonsurgical stage III NSCLC. Our results indicated that MTV60% was significantly associated with the PFS in nonsurgical stage III NSCLC. TLG50% was significantly correlated with OS and PFS in the cohort. However, our results suggested that SUVmax was not a reliable prognostic factor for survival in nonsurgical stage III NSCLC.
Notably, we found that MTV60% presented a significant correlation with the PFS (HR =0.402, P=0.042). In previous studies, MTV has been identified as a prognostic factor for survival but with various defined criteria (9,10,25,26). Bazan et al. demonstrated that pretreatment MTV was highly prognostic for OS and local-regional control (LC) in a group of uniformly treated patients with stage III NSCLC in a multi-institutional setting (25). In the investigation, the MTV was defined as all connected voxels with intensity greater than a lesion-specific adaptive threshold of 60% of the SUVpeak within the lesion (25). The SUVpeak within the ACRIN 6668 study was defined as the mean SUV within a circular ROI (0.75–1.5 cm in diameter) that encompasses the SUVmax (27). Lee et al. have reported that a high MTV50% is significantly associated with decreased OS in a subgroup of patients who were treated definitively (9). In another study, Hyun et al. demonstrated that MTV2.5 has been identified as an important prognostic factor for survival in stage IIIA NSCLC for patients treated with surgical resection (10). Our results are inconsistent with results from previous studies (9,10,20,26). We considered that there might be two reasons for this discrepancy. First, the previous studies used one of the standards of the MTV for the prognostic analysis, for instance, MTV2.5, or MTV50%, because it was used to approximate the TV (9,10,28). Furthermore, the previous studies did not perform comparative analysis among different levels of the MTV. In contrast, we analyzed a series of SUV thresholds of the MTV, comprising MTV2.5, MTV40%, MTV50%, MTV60%, MTV70%, MTV80%, and MTV90%. To some extent, our analysis may complement previous results. However, MTV60% is a TV fraction with a higher tumor metabolism than MTV2.5 or MTV50%. Some studies have indicated that the FDG uptake is positively correlated with the biological aggressiveness and prognosis in NSCLC (11,29). Hence, it is possible that MTV60% may represent a collection of more aggressive tumor cells than MTV40%, MTV50%, or MTV2.5, while MTV70%, MTV80% and MTV90% were relatively small in size, sometimes only a few voxels in PET/CT. Hence, for analyzing partial volume effects, their numerical values were not stable enough to serve as a prognostic factor.
In our previous study, we showed that the tumor sizes in CT are larger but similar to the pathology sizes (30). Kim et al. demonstrated that the lesion size of primary tumor, i.e., maximal tumor diameter as determined by pathological findings cannot predict recurrence in pN0 NSCLC patients who have undergone curative surgery (31). Soussan et al. reported that baseline CTV was not a prognostic factor in stage III NSCLC treated with induction chemotherapy (32). We obtained the similar conclusion that CTV was not a prognostic factor. The reason may be that the intratumoral heterogeneity of local advanced NCSLC would be more conspicuous, consequently, to some extent, CTV cannot reflect the real TV, for example, partial necrosis of tumor would be involved in the CTV. In the contrast, it was reasonable that MTV60% was a prognostic factor because it may represent the more aggressive sub-volumes of the tumor.
Soussan et al. used two segmentation methods, adaptive methods and fit methods, which were described respectively by Nestle et al. and Tylski et al., for tumor delineation in their studies (32,33). The two methods have been previously reported to be reliable and robust in a large variety of tumor configurations (34,35). Soussan et al. reported that TV and TLG ratios helped predict residual viable tumor to preoperative chemotherapy in locally advanced NSCLC (33). The strength of this study was the use of precise method for delineating tumor, thorough tumor sampling and analysis of all available slides to reliably determine the amount of residual tumor cells (33). Since insufficient conditions on in-house algorithms, regretfully, we did not use these methods to delineate the TV. Instead of that, we calculated MTVs automatically on the PET/CT images with the specified SUV and delineated the TV on the CT scan. To some extent, the relative threshold method for defining MTV possibly reflects the most metabolically active areas within each lesion (25), while the CTV represented an approximate size of TV. It was correspondent with our aim of the research, which was focused more on the prognostic value of the sub-volumes of the primary tumor compared with the whole TV.
According to our observations, the tumor shapes were variable and asymmetrical, and a high metabolic volume of the tumor was not always located in the center of the tumor, because the tumor composition and intratumoral 18F-FDG uptake are heterogeneous (3). AUC-CSH was known to reflect the tumor heterogeneity and lower AUC corresponding to higher degrees of heterogeneity (23). Kang et al. reported lower AUC-CSH could predict early disease progression in pretreatment FDG PET in inoperable stage III NSCLC (24). We did not obtain the consistent conclusion in our investigation. In our opinion, the most probably reason may be the larger proportion of primary tumors of T3/T4 in our cohort, and the small size of the sample. A large number of clinical trials and abundant basic research will be necessary in the future.
TLG combines volumetric and metabolic information and may more closely represent the tumor burden. Some studies have postulated that the summation of the tumor glycolysis, embodied by TLG, may be a good indicator for survival (26,36). Takahashi et al. showed that MTV was a prognostic factor for OS of patients with stage I lung cancer treated with SBRT. In the study, AUC values of SUVmax, MTV2, MTV4, MTV6, TLG40%, TLG50% and TLG60% for LC were 0.590, 0.645, 0.668, 0.608, 0.719, 0.701 and 0.687, respectively. The largest AUC for LC was TLG40%, followed by TLG50% and TLG60% (37). Satoh et al. have reported that TLG50% and TLG60% are significantly correlated with survival in early-stage NSCLC treated with SBRT, and this result has been found to be consistent when the tumor diameter is larger than 3 cm (8). Thus, we wondered whether TLG50% and TLG60% might have similar prognostic values for stage III NSCLC. Our results showed that TLG50% was a significant predictor of OS and PFS [HR =0.423, P=0.023; HR =0.457, P(TLG50%)=0.029]. As a prognostic factor, TLG is advantageous because of its simple and quick method of data collection. The results were obtained by a computer-based methodology, and the differences among observers were limited. These aspects enabled the efficient collection of tumor burden measurements (within approximately 1–2 min).
SUVmax as a prognostic factor for lung cancer has been subject to extensive research and debate (38-41). SUVmax has been found to be significantly related with NSCLC prognosis in some studies (38,40). Satoh et al. reported that SUVmax has been found to be associated with the prognosis when the diameter of the primary tumor is less than 3 cm in the early stage of NSCLC (8). Some studies have suggested that SUVmax is not an independent prognostic factor for NSCLC, especially in advanced lung cancer research (26,42). Lin et al. have demonstrated that in patients treated with radical RT for inoperable NSCLC, higher SUVmax derived from a staging FDG-PET/CT scan does not significantly correlate with poorer survival (43). We obtained a similar result in our paper: SUVmax was not an independent prognostic factor for nonsurgical stage III NSCLC in terms of the OS or PFS (HR =0.885, P=0.717; HR =0.928 P=0.809). We considered some possible reasons. SUVmax represents only the metabolic value of a point in the tumor lesions, and thus this parameter cannot represent the malignance degree or biological characteristics of the entire tumor. Moreover, the value is vulnerable to statistical noise.
Our research has several limitations that need to be acknowledged. First, this study was a retrospective analysis with a small sample size. Although the study included patients with stage III NSCLC exclusively, the chemo-radiotherapy regimens were not exactly the same, thus possibly confounding the analysis of the prognosis. Additionally, the results may be biased because of the relatively small sample size. Thus, a further prospective large-sample analysis is required. Second, the MTV values described in the current study are specific to the primary tumor, excluding the hilar or mediastinal metastatic lymph nodes, etc. Additionally, in some cases, the boundary between the primary tumor and the metastatic lymph nodes was not sufficiently clear. As a result, the MTV included the local metastatic lymph nodes at times. Finally, the partial volume effect of the PET/CT affected the analysis result to some extent. A few of the primary tumors were small; therefore, the higher metabolic volumes, such as MTV70%, MTV80% or MTV90%, were smaller. The results of the statistical analysis may thus have been affected to some degree.
Conclusions
Volume-based PET pretreatment parameters have prognostic value in nonsurgical stage III NSCLC. The prognosis analysis using different thresholds is important. A higher value of MTV60% predicts a worse PFS, and TLG50% has a negative correlation with both OS and PFS. These results would be helpful to identify proper cohorts for individualized therapeutic schedules, and need to be further investigated in larger cohorts in future trials.
Acknowledgments
Funding: This study was funded by the special fund for Scientific Research in the Public Interest (201402011).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.06.52). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Committee of the Cancer Hospital of Shandong Province, and all the patients signed informed consent (ID of Ethics Approval: 201208020).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Andre F, Grunenwald D, Pignon JP, et al. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol 2000;18:2981-9. [Crossref] [PubMed]
- Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:694-705. [Crossref] [PubMed]
- Berghmans T, Paesmans M, Sculier JP. Prognostic factors in stage III non-small cell lung cancer: a review of conventional, metabolic and new biological variables. Ther Adv Med Oncol 2011;3:127-38. [Crossref] [PubMed]
- Roy S, Pathy S, Kumar R, et al. Efficacy of 18F-fluorodeoxyglucose positron emission tomography/computed tomography as a predictor of response in locally advanced non-small-cell carcinoma of the lung. Nucl Med Commun 2016;37:129-38. [Crossref] [PubMed]
- Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. In: William D, Travis EB, Müller-Hermelink HK, et al. editors. World Health Organization Classification of Tumours. Lyon, France: IARC Press, 2004.
- Woodard GA, Jones KD, Jablons DM. Lung Cancer Staging and Prognosis. Cancer Treat Res 2016;170:47-75. [Crossref] [PubMed]
- Abelson JA, Murphy JD, Trakul N, et al. Metabolic imaging metrics correlate with survival in early stage lung cancer treated with stereotactic ablative radiotherapy. Lung Cancer 2012;78:219-24. [Crossref] [PubMed]
- Satoh Y, Onishi H, Nambu A, et al. Volume-based parameters measured by using FDG PET/CT in patients with stage I NSCLC treated with stereotactic body radiation therapy: prognostic value. Radiology 2014;270:275-81. [Crossref] [PubMed]
- Lee P, Bazan JG, Lavori PW, et al. Metabolic tumor volume is an independent prognostic factor in patients treated definitively for non-small-cell lung cancer. Clin Lung Cancer 2012;13:52-8. [Crossref] [PubMed]
- Hyun SH, Ahn HK, Kim H, et al. Volume-based assessment by (18)F-FDG PET/CT predicts survival in patients with stage III non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 2014;41:50-8. [Crossref] [PubMed]
- Higashi K, Ueda Y, Arisaka Y, et al. 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med 2002;43:39-45. [PubMed]
- Cooper RA, Carrington BM, Loncaster JA, et al. Tumour oxygenation levels correlate with dynamic contrast-enhanced magnetic resonance imaging parameters in carcinoma of the cervix. Radiother Oncol 2000;57:53-9. [Crossref] [PubMed]
- Busk M, Horsman MR, Jakobsen S, et al. Imaging hypoxia in xenografted and murine tumors with 18F-fluoroazomycin arabinoside: a comparative study involving microPET, autoradiography, PO2-polarography, and fluorescence microscopy. Int J Radiat Oncol Biol Phys 2008;70:1202-12. [Crossref] [PubMed]
- Shin Y, Han S, Chung E, et al. Intratumoral phenotypic heterogeneity as an encourager of cancer invasion. Integr Biol (Camb) 2014;6:654-61. [Crossref] [PubMed]
- Harpole DH Jr, Herndon JE 2nd, Young WG Jr, et al. Stage I nonsmall cell lung cancer. A multivariate analysis of treatment methods and patterns of recurrence. Cancer 1995;76:787-96. [Crossref] [PubMed]
- Macchiarini P, Fontanini G, Hardin MJ, et al. Blood vessel invasion by tumor cells predicts recurrence in completely resected T1 N0 M0 non-small-cell lung cancer. J Thorac Cardiovasc Surg 1993;106:80-9. [PubMed]
- Filderman AE, Silvestri GA, Gatsonis C, et al. Prognostic significance of tumor proliferative fraction and DNA content in stage I non-small cell lung cancer. Am Rev Respir Dis 1992;146:707-10. [Crossref] [PubMed]
- Volm M, Hahn EW, Mattern J, et al. Five-year follow-up study of independent clinical and flow cytometric prognostic factors for the survival of patients with non-small cell lung carcinoma. Cancer Res 1988;48:2923-8. [PubMed]
- Chicklore S, Goh V, Siddique M, et al. Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur J Nucl Med Mol Imaging 2013;40:133-40. [Crossref] [PubMed]
- Groheux D, Majdoub M, Tixier F, et al. Do clinical, histological or immunohistochemical primary tumour characteristics translate into different (18)F-FDG PET/CT volumetric and heterogeneity features in stage II/III breast cancer? Eur J Nucl Med Mol Imaging 2015;42:1682-91. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Yu J, Li X, Xing L, et al. Comparison of tumor volumes as determined by pathologic examination and FDG-PET/CT images of non-small-cell lung cancer: a pilot study. Int J Radiat Oncol Biol Phys 2009;75:1468-74. [Crossref] [PubMed]
- van Velden FH, Cheebsumon P, Yaqub M, et al. Evaluation of a cumulative SUV-volume histogram method for parameterizing heterogeneous intratumoural FDG uptake in non-small cell lung cancer PET studies. Eur J Nucl Med Mol Imaging 2011;38:1636-47. [Crossref] [PubMed]
- Kang SR, Song HC, Byun BH, et al. Intratumoral Metabolic Heterogeneity for Prediction of Disease Progression After Concurrent Chemoradiotherapy in Patients with Inoperable Stage III Non-Small-Cell Lung Cancer. Nucl Med Mol Imaging 2014;48:16-25. [Crossref] [PubMed]
- Bazan JG, Duan F, Snyder BS, et al. Metabolic tumor volume predicts overall survival and local control in patients with stage III non-small cell lung cancer treated in ACRIN 6668/RTOG 0235. Eur J Nucl Med Mol Imaging 2017;44:17-24. [Crossref] [PubMed]
- Liao S, Penney BC, Wroblewski K, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2012;39:27-38. [Crossref] [PubMed]
- Machtay M, Duan F, Siegel BA, et al. Prediction of survival by [18F]fluorodeoxyglucose positron emission tomography in patients with locally advanced non-small-cell lung cancer undergoing definitive chemoradiation therapy: results of the ACRIN 6668/RTOG 0235 trial. J Clin Oncol 2013;31:3823-30. [Crossref] [PubMed]
- Yoo SW, Kim J, Chong A, et al. Metabolic Tumor Volume Measured by F-18 FDG PET/CT can Further Stratify the Prognosis of Patients with Stage IV Non-Small Cell Lung Cancer. Nucl Med Mol Imaging 2012;46:286-93. [Crossref] [PubMed]
- Vansteenkiste JF, Stroobants SG, Dupont PJ, et al. Prognostic importance of the standardized uptake value on (18)F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: An analysis of 125 cases. Leuven Lung Cancer Group. J Clin Oncol 1999;17:3201-6. [Crossref] [PubMed]
- Yu HM, Liu YF, Hou M, et al. Evaluation of gross tumor size using CT, 18F-FDG PET, integrated 18F-FDG PET/CT and pathological analysis in non-small cell lung cancer. Eur J Radiol 2009;72:104-13. [Crossref] [PubMed]
- Kim DH, Jung JH, Son SH, et al. Prognostic Significance of Intratumoral Metabolic Heterogeneity on 18F-FDG PET/CT in Pathological N0 Non-Small Cell Lung Cancer. Clin Nucl Med 2015;40:708-14. [Crossref] [PubMed]
- Soussan M, Chouahnia K, Maisonobe JA, et al. Prognostic implications of volume-based measurements on FDG PET/CT in stage III non-small-cell lung cancer after induction chemotherapy. Eur J Nucl Med Mol Imaging 2013;40:668-76. [Crossref] [PubMed]
- Soussan M, Cyrta J, Pouliquen C, et al. Fluorine 18 fluorodeoxyglucose PET/CT volume-based indices in locally advanced non-small cell lung cancer: prediction of residual viable tumor after induction chemotherapy. Radiology 2014;272:875-84. [Crossref] [PubMed]
- Nestle U, Kremp S, Schaefer-Schuler A, et al. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-Small cell lung cancer. J Nucl Med 2005;46:1342-8. [PubMed]
- Tylski P, Stute S, Grotus N, et al. Comparative assessment of methods for estimating tumor volume and standardized uptake value in (18)F-FDG PET. J Nucl Med 2010;51:268-76. [Crossref] [PubMed]
- Chen HH, Chiu NT, Su WC, et al. Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non-small cell lung cancer. Radiology 2012;264:559-66. [Crossref] [PubMed]
- Takahashi N, Yamamoto T, Matsushita H, et al. Metabolic tumor volume on FDG-PET/CT is a possible prognostic factor for Stage I lung cancer patients treated with stereotactic body radiation therapy: a retrospective clinical study. J Radiat Res 2016;57:655-61. [Crossref] [PubMed]
- Sasaki R, Komaki R, Macapinlac H, et al. [18F]fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol 2005;23:1136-43. [Crossref] [PubMed]
- Vesselle H, Freeman JD, Wiens L, et al. Fluorodeoxyglucose uptake of primary non-small cell lung cancer at positron emission tomography: new contrary data on prognostic role. Clin Cancer Res 2007;13:3255-63. [Crossref] [PubMed]
- Paesmans M, Berghmans T, Dusart M, et al. Primary tumor standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: update of a systematic review and meta-analysis by the European Lung Cancer Working Party for the International Association for the Study of Lung Cancer Staging Project. J Thorac Oncol 2010;5:612-9. [Crossref] [PubMed]
- Lee P, Weerasuriya DK, Lavori PW, et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Oncol Biol Phys 2007;69:328-33. [Crossref] [PubMed]
- Chung HW, Lee KY, Kim HJ, et al. FDG PET/CT metabolic tumor volume and total lesion glycolysis predict prognosis in patients with advanced lung adenocarcinoma. J Cancer Res Clin Oncol 2014;140:89-98. [Crossref] [PubMed]
- Lin MY, Wu M, Brennan S, et al. Absence of a relationship between tumor (1)(8)F-fluorodeoxyglucose standardized uptake value and survival in patients treated with definitive radiotherapy for non-small-cell lung cancer. J Thorac Oncol 2014;9:377-82. [Crossref] [PubMed]


