The polymorphism of JAK2 rs56118985 may be a predictive marker of the treatment responses of acute myeloid leukemia patients
Introduction
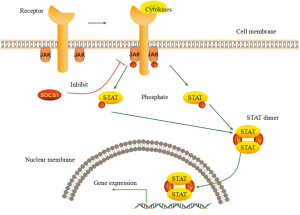
The Janus kinase/Signal transducer and activator of transcription (JAK/STAT) signaling pathway is activated by a variety of cytokines, hormones and growth factors. Ligand binding to cell surface receptors triggers JAK kinases, which activate JAK-mediated phosphorylation and STAT protein dimerization and translocate to the nucleus, where they regulate gene expression (Figure 1) (1). The JAK/STAT signaling pathway is a major signal transduction pathway in cytokine and growth factor signaling, which controls the survival, proliferation and differentiation of several cell types. Aberrant JAK/STAT signaling has been shown to be involved in tumorigenesis and the progression of several solid cancers, as well as hematopoietic malignancies. Recently, hematologists have turned their attention to mutations and single nucleotide polymorphisms (SNPs) of JAK2, an important member of the signaling pathway. An acquired somatic mutation in JAK2 was identified in a majority of patients with myeloproliferative disorders (MPN). The V617F mutation drives the proliferation of neoplastic clones and promotes JAK2 catalytic subunit activation and cytokine-independent signaling (2,3). In addition, in a study of V617F-negative polycythemia vera (PV) patients, Scott et al found that JAK2 exon 12 mutations may play an important role in the progression of PV (4). Currently, association studies of SNPs of the JAK/STAT pathway and the risk of hematologic malignancies are ongoing. Animesh’s group reported that three JAK2 SNPs (rs7046736, rs10815148 and rs12342421) were significantly associated with PV and essential thrombocythemia (ET) by genotype-phenotype analysis of 179 Caucasian patients [84 with PV, 58 with primary myelofibrosis (PMF) and 37 with ET]. Pardanani et al. found three other JAK2 SNPs (rs10758669, rs3808850, and rs10974947) that were also significantly associated with PV (5).
Acute myeloid leukemia (AML) is a heterogeneous group of leukemia that results from clonal transformation of hematopoietic precursors through the acquisition of chromosomal rearrangements and multiple gene mutations. Chemotherapy has been the backbone of treatment for AML. At present, more than 50% of adults with AML achieve a complete response (CR) following induction therapy with cytosine arabinoside (Ara-C)-based chemotherapy (6,7). The cause of resistance to chemotherapy has confounded hematologists. Currently, SNPs are popularly used as molecular markers to facilitate the identification of genetic factors responsible for diseases, as well as the early diagnosis and prediction of treatment outcome (8,9). Some genes have been reported to be associated with the response to chemotherapy in AML patients (10-13). Thus far, the ideal candidate gene predicting the response of AML patients to chemotherapy response has still not been identified.
In this retrospective study, we aimed to investigate whether the genetic polymorphism rs56118985 (Glycine ⇒ Aspartic acid), located at exon 5 of the JAK2 gene, is associated with the therapeutic outcomes of Ara-C-based chemotherapy in AML patients.
Methods
Patients
A total of 552 patients with newly diagnosed AML according to the World Health Organization (WHO) and French-American-British (FAB) criteria in the Drum Tower Hospital and other four large hospitals in Jiangsu Province between August 2007 and January 2014 were enrolled in the study. This study was approved by the ethics committees of all participating hospitals and was performed in accordance with the Declaration of Helsinki. All patients provided written informed consent before enrollment and were informed of the existence of other treatment options. Patients who were diagnosed with any other type of cancer or other hematological malignancies were excluded from the study. Patients with the M3 subtype (acute promyelocytic leukemia) and patients who underwent allogeneic hematopoietic stem cell transplantation (HSCT) or autologous HSCT were also excluded. Cytogenetic risk groups were stratified according to the Medical Research Council cytogenetic classification system (14). The presence of 5/del(Jeny5q), 7/del(Jeny7q), abn3q, complex aberrations (≥3 independent aberrations), t(Jeny9;Jeny22), and t(Jeny6;Jeny9) were identified as unfavorable karyotypes, whereas t(Jeny8;Jeny21) and inv(Jeny16) were classified as favorable karyotypes. Gene mutation risk groups were divided according to the NCCN gene mutation classification system (15). NPM1 and CEBPA were identified as favorable gene mutations, whereas FLT3/ITD and C-KIT were classified as unfavorable gene mutations. The remaining patients with normal karyotypes and patients with other karyotypic aberrations comprised the intermediate risk group. Patients were further divided into two groups according to their white blood cell (WBC) counts, hemoglobin levels and platelet counts.
Chemotherapy regimens and evaluation of the therapeutic effect
All patients enrolled were treated with standard Ara-C-based chemotherapy regimens; DA induction chemotherapy regimens, Daunorubicin (DNR) 60 mg/m2/day for 1–3 days and Ara-C 100 mg/m2/day for 1–7 days; HA induction chemotherapy regimens, homoharringtonine (HHT) 3–4 mg/m2/day for 5–7 days and Ara-C 100 mg/m2/day for 1–7 days; and MA induction chemotherapy regimens, Mitoxantrone 4 mg/m2/day for 1–5 days and Ara-C 100 mg/m2/day. All chemotherapeutic drugs were administered intravenously. Complete remission (CR) was defined according to the criteria of the International Working Group (15). Partial remission (PR) indicates that at least one of the standards of clinical manifestation, blood analysis and bone marrow was not met. Additionally, blast cells and promyelocytic cells should be less than 20% in the bone marrow. Non-remission (NR) indicates that clinical manifestation, blood analysis and bone marrow did not meet the standards for CR. Additionally, promyelocytic cells should be more than 20% in the bone marrow. Early death was defined as death within 8 weeks from the start of the first induction therapy course. Progression-free survival (PFS) was defined as the interval from the date of treatment to the date of confirmed relapse or death from any cause. Overall survival (OS) was defined as the length of time from the date of diagnosis to the date of death from any cause or the last follow-up.
DNA extraction and polymorphism genotyping
Genomic DNA was isolated from peripheral blood leukocytes using the phenol-chloroform extraction method. SNP genotyping was performed by the MassARRAY system (Sequenom, USA) with matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF-MS) according to the manufacturer’s instructions.
Statistical analysis
Demographic and clinical variables of different genotypes were evaluated using the Pearson Chi-squared test or Fisher’s exact test, as appropriate. The Kaplan–Meier product-limit method was used to determine OS and PFS, and the differences were assessed by the log-rank test. The prognostic impact of different variables on survival (OS and PFS) was determined by multivariate Cox proportional hazards model. All data were analyzed statistically using a commercially available statistical software package (SPSS 19.0; IBM Corp., Armonk, NY, USA). All tests were two-tailed, and a P value <0.05 was considered statistically significant. Odds ratios (ORs) with 95% confidence intervals (CIs) were also calculated.
Results
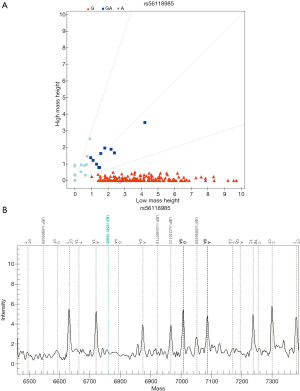
Genotyping by MALDI-TOF MS
A typical MALDI-TOF MS spectrum after mini sequencing by standard ddNTPs and a corresponding scatter plot are shown in Figure 2.
Patient and SNP genotype characteristics
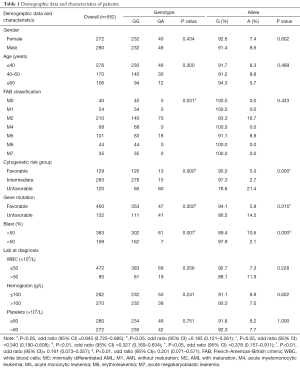
We found that genotype frequencies for the polymorphisms did not deviate from Hardy-Weinberg equilibrium, and no individuals were detected with the AA genotype in JAK2 rs56118985 among AML patients in the Chinese population. Patient characteristics are listed in Table 1. The median follow-up was 24 months (range 5–54 months). The male-to-female ratio was 0.97:1 (272:280), with ages ranging from 14 to 91 years, and the median age of the AML patients at diagnosis was 47 years. No significant difference was identified with regard to age, gender and FAB classification in patients with different JAK2 genotypes in rs56118985. The results of the analysis of cytogenetic risk showed that the frequency of the GG genotype was significantly higher in the intermediate group than in the other two groups, whereas the GA genotype was observed more in the unfavorable group (P<0.01). Moreover, patients with M5, unfavorable gene mutation factor, and high ratio of blast were associated with the GA genotype. (P=0.031, P=0.000, P=0.000, and P=0.007, respectively). Meanwhile, the A allele in rs56118985 was more frequently associated with the unfavorable group, the unfavorable gene mutation factor group, and high ratio of blast group (P<0.01, P=0.015, and P=0.000, respectively). We then stratified the patients into two groups according to their WBC counts, hemoglobin levels and platelet counts, the results were similar in patients with different JAK2 rs56118985 genotypes (P>0.05).
Full table
Treatment outcomes in relation to genetic polymorphism
All 552 patients were administered Ara-C-based standard induction chemotherapy regimens [DA (daunorubicin and cytosine arabinoside), MA (mitoxantrone and cytosine arabinoside), HA (homoharingtonine and cytosine arabinoside)]. Patient responses to treatment were evaluated after every cycle of chemotherapy, which was subdivided into four groups, namely CR, PR, NR, and early death. Differences in responsiveness to chemotherapy on the basis of the JAK2 rs56118985 genotypes are shown in Table 2. There were 260 cases that achieved CR after the first course of treatment and 87 cases that achieved CR after the second course. We found statistically significant association between the GA and GG genotypes in the CR and NR patients. No differences were identified between the two genotypes (GA and GG) in PR. A statistically significant association was found between the GA and GG genotypes with respect to the percentage of early death. The patients with the GA genotype suffered from a higher early death rate (P<0.01). As reflected in the statistical analyses, more AML patients with the GG genotype in rs56118985 achieved a good response than patients with the GA genotype (P<0.01).

Full table
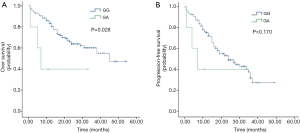
The Kaplan-Meier method and log-rank test show that patients with the GG genotype in rs56118985 had significantly better OS than those with the GA genotype (P=0.028, with a 24-month OS of 62.5% versus 40.0%) (Figure 3A). The 24-month PFS rates of patients with the GG and GA genotype were 42.5% and 40.0%, respectively. Patients with GA genotype did not have significantly poorer PFS than those with the GG genotype (P=0.170) (Figure 3B).
The survival analyses by Cox regression model are shown in Table 3. On univariate analysis, FAB classification (P=0.020, P=0.036), CR after 1st Course (P=0.018, P=0.034), cytogenetic risk (P=0.027, P=0.003), gene mutation (P=0.001, P=0.001), and rs56118985GA/GG genotype (P=0.013, P=0.021) were significantly associated with EFS and PFS. However, on multivariate Cox regression analysis, only cytogenetic risk (P=0.048, P=0.046, respectively) and r rs56118985GA/GG genotype (P=0.015, P=0.039, respectively) remained as an independent significant predictor for EFS and PFS.
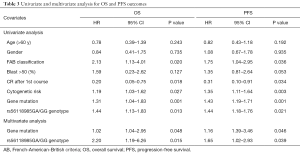
Full table
Discussion
In the present study, we found that the polymorphisms of JAK2 rs56118985 were significantly associated with risk status and prognosis of AML patients. Patients with GA genotype tended to have higher risk status, lower response rate to Ara-C based chemo-regimen, higher early death rate and shorter OS. With multivariate Cox regression analysis, we revealed that JAK2 rs56118985 GA/GG genotype was an independent predictor for EFS and PFS in AML. In this retrospective study in which Ara-C-based standard chemotherapeutic regimens were used to treat AML patients, the predictive role of JAK2 rs56118985 was shown to be clinically important. It suggested that JAK2 rs56118985 genotype could be used as an important predictive marker for the prognosis of AML patients. Considering that patients who receive allo or auto-hematopoietic stem cell transplantation have quite different prognosis from those who only receive chemotherapy, we excluded the AML patients who underwent stem cell transplantation in our study. Since the number of patients who received stem cell transplantation was small compared with the study population, we do not think that this selection would cause significant bias to our results.
The prevalence of patients with AML is the highest in leukemia patients, with 3.8 cases per 100,000 adults rising to 17.9 cases per 100,000 adults aged 65 years and older (16). It is a great challenge for hematologists to control the occurrence and development of AML. A majority of studies regarding the association of SNPs with AML have examined disease susceptibility (17). SNPs can also contribute to the diversity of drug tolerances and differences in responses to environmental factors. In the present study, we investigated the association between chemotherapy outcomes, as well as survival outcomes, and JAK2 rs56118985 in the AML patients for the first time. Like JAK2 V617F, many of the JAK2 mutations affect amino acids located within the pseudokinase domain JH2, which result in over-activation of the JAK-STAT pathway (18-22). It may also be the mechanism behind the association between JAK2 rs56118985 polymorphism and AML patient prognosis, but further studies are required to testify the hypothesis.
In summary, we found that the rs56118985 polymorphism of JAK2 was associated with the response of Chinese AML patients to Ara-C-based standard induction chemotherapy regimens. However, well-designed, larger studies will be necessary to further define the role and value of this polymorphism in the conventional treatment setting for patients with AML. We also look forward to the development of potential novel target therapies with the clinical application of SNPs.
Acknowledgments
We would like to thank all the volunteers who took part in this study.
Funding: This work was supported by the National Natural Science Foundation of China (81400162, 81570174), the Jiangsu Provincial Medical Innovation Team (CXTDA2017046), the Jiangsu Provincial Medical Youth Talent (QNRC2016039), the Technique Development Foundation of Nan Jing (YKK16099).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.06.40). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the ethics committees of all participating hospitals and was performed in accordance with the Declaration of Helsinki. All patients provided written informed consent before enrollment and were informed of the existence of other treatment options.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Darnell JE Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994;264:1415-21. [Crossref] [PubMed]
- James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144-8. [Crossref] [PubMed]
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054-61. [Crossref] [PubMed]
- Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007;356:459-68. [Crossref] [PubMed]
- Pardanani A, Fridley BL, Lasho TL, et al. Host genetic variation contributes to phenotypic diversity in myeloproliferative disorders. Blood 2008;111:2785-9. [Crossref] [PubMed]
- Büchner T, Hiddemann W, Wormann B, et al. Double induction strategy for acute myeloid leukemia: the effect of high-dose cytarabine with mitoxantrone instead of standard-dose cytarabine with daunorubicin and 6-thioguanine: a randomized trial by the German AML Cooperative Group. Blood 1999;93:4116-24. [PubMed]
- Bolaños-Meade J, Guo C, Gojo I, et al. A phase II study of timed sequential therapy of acute myelogenous leukemia (AML) for patients over the age of 60: two cycle timed sequential therapy with topotecan, ara-C and mitoxantrone in adults with poor-risk AML. Leuk Res 2004;28:571-7. [Crossref] [PubMed]
- Diabetes Genetics Initiative of Broad Institute of Harvard and MIT. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science (New York, NY) 2007;316:1331-6. [Crossref] [PubMed]
- Fang HM, Tian G, Zhou LJ, et al. FGFR4 genetic polymorphisms determine the chemotherapy response of Chinese patients with non-small cell lung cancer. Acta Pharmacol Sin 2013;34:549-54. [Crossref] [PubMed]
- Zhe N, Wang J, Chen S, et al. Heme oxygenase-1 plays a crucial role in chemoresistance in acute myeloid leukemia. Hematology (Amsterdam, Netherlands) 2015;20:384-91. [PubMed]
- van den Heuvel-Eibrink MM, van der Holt B, Burnett AK, et al. CD34-related coexpression of MDR1 and BCRP indicates a clinically resistant phenotype in patients with acute myeloid leukemia (AML) of older age. Ann Hematol 2007;86:329-37. [Crossref] [PubMed]
- Damiani D, Tiribelli M, Franzoni A, et al. BAALC overexpression retains its negative prognostic role across all cytogenetic risk groups in acute myeloid leukemia patients. Am J Hematol 2013;88:848-52. [Crossref] [PubMed]
- Barragan E, Collado M, Cervera J, et al. The GST deletions and NQO1*2 polymorphism confers interindividual variability of response to treatment in patients with acute myeloid leukemia. Leuk Res 2007;31:947-53. [Crossref] [PubMed]
- Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010;116:354-65. [Crossref] [PubMed]
- Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003;21:4642-9. [Crossref] [PubMed]
- Estey E, Dohner H. Acute myeloid leukaemia. Lancet 2006;368:1894-907. [Crossref] [PubMed]
- Zhong Y, Wu J, Ma R, et al. Association of Janus kinase 2 (JAK2) polymorphisms with acute leukemia susceptibility. Int J Lab Hematol 2012;34:248-53. [Crossref] [PubMed]
- Kratz CP, Boll S, Kontny U, et al. Mutational screen reveals a novel JAK2 mutation, L611S, in a child with acute lymphoblastic leukemia. Leukemia 2006;20:381-3. [Crossref] [PubMed]
- Lee JW, Kim YG, Soung YH, et al. The JAK2 V617F mutation in de novo acute myelogenous leukemias. Oncogene 2006;25:1434-6. [Crossref] [PubMed]
- Zhang SJ, Li JY, Li WD, et al. The investigation of JAK2 mutation in Chinese myeloproliferative diseases-identification of a novel C616Y point mutation in a PV patient. Int J Lab Hematol 2007;29:71-2. [PubMed]
- Schnittger S, Bacher U, Kern W, et al. Report on two novel nucleotide exchanges in the JAK2 pseudokinase domain: D620E and E627E. Leukemia 2006;20:2195-7. [Crossref] [PubMed]
- Malinge S, Ben-Abdelali R, Settegrana C, et al. Novel activating JAK2 mutation in a patient with Down syndrome and B-cell precursor acute lymphoblastic leukemia. Blood 2007;109:2202-4. [Crossref] [PubMed]