
Effectiveness of digital breast tomosynthesis (3D-mammography) in population breast cancer screening: a protocol for a collaborative individual participant data (IPD) meta-analysis
Introduction
Evidence on digital breast tomosynthesis (3D-mammography) for population breast screening has accumulated in recent years (1-6). Prospective screening trials (1,3,5-8) and retrospective evaluations (2,9-13) have shown that screening using tomosynthesis with (or instead of) standard 2D digital mammography improves screen-detection measures compared to standard 2D-mammography. Studies evaluating cancer detection measures for tomosynthesis (hereafter ‘3D-mammography’) screening are ongoing, to assess or to confirm the enhanced cancer detection rates observed in the pivotal trials (1,3,5-8) in new studies or in program-based evaluations (for example, search of ClinicalTrials.gov at February 2017 shows at least ten trials of 3D-mammography screening are recruiting or recently completed). However the most important gap in evidence relates to the effectiveness of 3D-mammography screening: it is unknown whether the additional cancer detection from 3D-mammography leads to incremental screening benefit or whether it is mostly over detecting (indolent) breast cancers. An appropriate method to investigate this issue is to conduct large RCTs that examine 10–15-year mortality outcomes however this approach is not feasible in most settings, and cannot address the urgently needed evidence to guide screening policy and recommendations in the foreseeable future.
An alternate informative approach is to investigate the impact of 3D-mammography on interval cancer rates: an interval cancer is a cancer that presents after a ‘negative’ screening examination and before the next scheduled screen, in other words a cancer that arises or is diagnosed in the inter-screening interval. If an increase in breast cancer detection through 3D-mammography screening leads to a subsequent reduction in interval cancers, then this would provide evidence that the additional cancers detected do not preferentially represent over-diagnosis. That is, 3D-mammography screening will have averted cancers from progressing to clinically-presenting disease. It would also provide direct evidence that 3D-mammography screening enhances screening program sensitivity.
This critical evidence gap relating to impact on interval cancer rates is unlikely to be addressed by any individual published study because (I) the individual studies were not designed or powered to examine interval cancer rate reductions as an end-point; (II) studies based on annual breast screening are unlikely to be informative because the majority of interval cases occur in the latter half of the inter-screening interval in biennial screening; and (III) the existing prospective trials screened all women with both 3D-mammography and 2D-mammography (reporting paired comparative data for each screened participant) hence future reported data on interval cancer rates from these studies will come from cohorts screened with 3D-mammography (lacking a comparator or control cohort). However, these 3D-mammography screening trials can contribute to the proposed IPD meta-analysis, provided additional work is undertaken to form appropriate comparison cohorts screened with 2D-mammography alone.
This proposed collaboration comprises an IPD meta-analysis research strategy that includes a framework for research linked to existing (published) studies, to address the evidence gap on the effect of 3D-mammography on interval cancer rates. By addressing this critical evidence gap we expect to underpin translation of 3D-mammography technology into breast screening practice. The aim of the proposed research is to examine whether 3D-mammography population screening improves breast screening effectiveness by reducing interval cancer rates, compared to standard digital (2D) mammography screening, using IPD meta-analysis.
Methods
An IPD meta-analysis will be performed using the following combined approach, also shown in Figure 1:
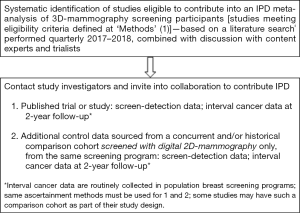
- Systematic identification of studies eligible to contribute data into an IPD meta-analysis of 3D-mammography screening participants, sourced from prospective trials comparing 3D-mammography screening (3D alone or integrated 2D/3D or 2Dsynthetic/3D) with 2D-mammography in biennial screening practice, and reporting breast cancer detection measures. Preliminary identification of eligible studies based on literature searching (performed July 2016) will be supplemented by regular (quarterly) updated searches and discussion with trialists and content experts, and has guided formation of the present collaborating team. Eligible studies identified in the preliminary search are briefly described in Table 1. Collaborators who have indicated in-principle agreement to contribute to this project will have the opportunity to provide input into the development of the research at every stage of the proposed work.
 Table 1 Prospective trials evaluating 3D-mammography (digital breast tomosynthesis) for population breast screening
Table 1 Prospective trials evaluating 3D-mammography (digital breast tomosynthesis) for population breast screening
Full table - Additional data request from studies identified as eligible in (1) to provide IPD for a comparison cohort, assembled from concurrent or historical controls, or both, from the same screening program, screened with digital 2D-mammography only. It is anticipated that some studies may have such a comparison cohort as part of their study design, and if not, we will recommend that the comparison cohort be from the same program and also the same screening service(s).
The above-described approach has been conceived to enable estimation of:
- Differences in interval cancer rates (3D-mammography vs. standard screening);
- Differences in screening program sensitivity inclusive of interval cases (3D-mammography vs. standard screening);
- Characterization of the cancers detected at 3D-mammography based on histopathology (within trial comparisons using paired data; and comparison versus standard screening cohorts).
Study eligibility criteria—studies to be included in the IPD meta-analysis will meet pre-defined eligibility criteria as follows:
- Population breast cancer screening studies investigating 3D-mammography (interpreted alone, or in conjunction with acquired or synthetic 2D-images) in comparison with standard (2D) mammography for screening;
- Use a prospective design (prospective recruitment of screening participants into the study), hence prospective trials or prospective cohort studies;
- Report (at minimum) data on breast cancer detection: number of cancers and number of screens and/or cancer detection rates;
- Have a biennial screening interval (or predominantly biennial, meaning that <25% of subjects received annual or 18-monthly screening). This screening interval criterion was chosen to ensure that any expected effect of 3D-mammography on interval cancer rates (primary endpoint) is not diluted through frequent (annual or 18-monthly) screening, and to be commensurate with practice in organized population-based breast screening programs.
Exclusion criteria:
- Retrospective design;
- Studies based on annual screening;
- Studies based on participants classified as having increased risk of breast cancer (studies that are selecting women based on specified risk factors and hence not performing population screening), or based on symptomatic women.
Intervention: digital breast tomosynthesis technology, also referred to as 3D-mammography, used for screening or screen-reading, alone or integrated with 2D-mammography (2D/3D) or with synthetic 2D-mammography (2D synthetic/3D); this may comprise one view or two view 3D-mammography acquisitions.
Comparison: digital 2D-mammography screening only (standard of care in breast screening).
Participants: women (≥40 years) presenting for routine mammography screening.
Data collection and management
Investigators from eligible studies will be contacted and provided with the research plan, and those with intention to collaborate in this work will be requested to provide IPD according to pre-agreed timelines, and to also plan the work on sourcing a comparison cohort (if not already undertaken or in progress). Trial investigators will provide de-identified IPD in any convenient format by electronic transfer of password-protected database. Individual trial data will be stored in a custom-designed secure database accessible only by research staff (based at University of Sydney, Sydney, Australia) directly responsible for checking, managing, and analyzing the collective database. Recoding of data is expected to be minimal for this project however, where undertaken, will be verified by the trialists.
Data checks will be conducted for range of values, missing or extreme values, and also analyzed at the aggregate level for cross-checking against published study reports. Inconsistencies or missing data will be discussed with individual investigators for each study to resolve problems by consensus or by further data checking (or both). Each study will be checked individually and sent to the responsible trialist or representative investigator for verification. The same approach will be used for comparison cohorts. Further details regarding data management will be discussed and documented at collaborators’ meetings.
Definition and ascertainment of interval cancers
Definition and ascertainment methods for interval breast cancers vary between screening settings (14) and programs, hence may also vary between studies: our provisional definition as shown at study-level variables in this proposal will be reviewed and standardised for analytic purposes allowing for study-specific definitions to be collected from contributing studies (see ‘study-level variables’). Because most screening services that use biennial screening as part of organized programs generally have quality assurance standards that necessitate monitoring of interval cancer data, we anticipate that contributing studies will have existing processes to ascertain interval cancers. As there may be heterogeneity between ascertainment methods, we are obtaining information on these methods (see ‘study-level variables’). Our analytic plans may incorporate a sensitivity analysis to assess whether heterogeneity in interval cancer ascertainment methods or definitions across studies (if present) alters primary endpoint results.
Data items to be collected for IPD meta-analysis
Study-level variables
- Country (region)
- Number of screening participants
- Number (or proportion) who had annual or 18-monthly screens (if any)
- Screening start and finish dates for the study
- Informed consent method
- Ethics approval (entity or institutional body providing approval)
- 3D-mammography intervention (technology/unit, number of views)
- Method of reading (single or double-reading)
- Number of readers contributing to screen-readings in the study
- Method or rule for deciding recall (including scoring used if applicable)
- Method or rule for resolving discordant double-reading (where applicable)
- Method for classifying breast density
- Method of ascertaining interval cancers [cancer registry linkage; hospital or clinical records linkage; other (details to be provided)]
- Study-specific definition of interval cancers
- The same study-level data items will be requested for the comparison cohort, except that the intervention (by definition of standard of care in screening) is two-view digital mammography.
Participant-level variables
- Unique identification code
- Date of birth/age at screen
- Screen date
- Screen round (first or repeat)
- Breast density category
- Screen result by mammography modality (data for double-read as actioned in study, inclusive of resolved discordance where applicable):
- Screen-reading modality;
- Recall (positive)/no recall (negative);
- Other recall (recalled because of a reported symptom and not for screen-detected finding).
- For recalled screens only:
- Outcome (breast cancer/no breast cancer); cases with lobular carcinoma in situ only will be classified as ‘no breast cancer’.
- For negative (non-recalled) screens or recalled screens with outcome of ‘no breast cancer’—record of a breast cancer diagnosis or interval cancer notification within up to 2-year follow-up from screen date:
- Interval cancer (defaults to ‘no’ unless recorded as interval case);
- Date cancer diagnosis recorded (defaults to ‘no’ unless recorded as interval case).
The above data for interval cancers diagnosed within up to 2 years from screen data will not count breast cancers that are screen-detected in women returning earlier (20–24 months) for their biennial screen.
- For breast cancer only:
- Histological type;
- Tumour size (mm);
- Tumour grade;
- Axillary node status (for invasive cancer);
- Biomarker data (ER/PR, HER2, ki-67 where available) (for invasive cancer).
Analysis plan
Planned analyses will be developed inclusive of a detailed statistical plan following collaborators’ planning meetings, and pending annual literature searches to identify additional studies that are potentially eligible to contribute into this research (see Methods). This protocol outlines only the preliminary analysis plan. Analyses will be based on the checked and updated IPD from all available studies, performed in two stages because of the delay inherent in obtaining interval cancer data: initial stage analyses will be based on screen-detection data, and will precede the subsequent stage of analyses that includes interval cancer data as the primary end-point of this work.
Descriptive analyses will be performed to summarize study-specific characteristics (defined at study level variables) and participant level characteristics, namely age and breast density distributions for 3D-mammography-screened and 2D-mammography-screened cohorts.
Analysis of primary outcome of IPD meta-analysis
Descriptive data for interval breast cancers occurring within two-years from screen date (number of cancers; median time from screen to interval cancer diagnosis) will be calculated for 3D-mammography screening and for 2D-mammography screening. Interval breast cancer rates per 10,000 screens will be calculated and compared for 3D-mammography screening (3D or integrated 2D/3D or 2Dsynthetic/3D) and for standard 2D-mammography screening: the reduction in interval cancer rates (per 10,000 screens) in association with 3D-mammography screening will be estimated with 95% CI. The number of additional (‘extra’) cancers detected in the 3D-mammography screening cohorts, relative to comparison cohorts, that are needed to avert (reduce) one interval cancer will be calculated. Sensitivity analysis may be performed to assess whether heterogeneity in interval cancer ascertainment methods or definitions across studies (if any) alters results.
Screening sensitivity will be calculated and compared for 3D-mammography screening and for 2D-mammography screening, using data for screen-detected cancers (see secondary endpoints) and data for interval cancers, as follows: Sensitivity % = number of screen-detected cancers/(number of screen-detected + interval cancers).
The above-described analyses will also be reported by age-group and density strata. If there are sufficient studies reporting data for 2Dsynthetic/3D-mammography screening, then results may also be stratified according to whether 2Dsynthetic/3D or 2D/3D was used.
Secondary endpoints
Initial stage analysis will examine screening detection measures, and also histopathologic breast cancer characteristics, for 3D-mammography screening and for 2D-mammography screening, for the following comparative analyses:
- Paired data comparisons: these will be based on within participant comparisons of data for screen-reading with 2D-mammography alone and subsequent or parallel readings with 3D-mammography (3D or integrated 2D/3D or 2Dsynthetic/3D) for the same participants at the same screening episode—hence these analyses will be commensurate with the comparisons undertaken in the original prospective studies;
- Independent groups comparisons: these will be based on comparisons of cohorts screened with 2D only and cohorts screened with 3D-mammography (3D or 2D/3D or 2Dsynthetic/3D)—hence these analyses will compare the trial cohorts with the comparison cohorts.
Comparative analyses of secondary endpoints [as outlined above in (a) and (b)] will examine:
- Number of detected cancers, and the cancer detection rate (CDR) per 1,000 screens (and 95% CI);
- Differences in CDR per 1,000 screens between 3D-mammography and 2D-mammography screening;
- Cancer characteristics based on histopathology (distributions for tumour histology, size, and grade, and biomarkers; lymph node status);
- The number and percentage of recall and false-positive recall;
- The positive predictive value (PPV) for recall.
Analyses will also be reported by age-group and density strata. If there are sufficient studies reporting data for 2Dsynthetic/3D-mammography screening, then results may also be stratified according to whether 2Dsynthetic/3D or 2Dacquired/3D was used (otherwise this will be addressed in sensitivity analysis).
Sensitivity analyses will be undertaken for secondary endpoints to assess (I) effect of excluding data for 3D-mammography-only screening (stand-alone 3D-mammography, or 3D-mammography only acquisitions); and (II) effect of excluding/including the subset of women who reported a breast symptom at the screening episode.
Ethics and project management
Participants in each individual prospective trial or study had provided consent to participate in each respective study. For the comparison cohort, appropriate ethics approval will be required from each contributing trial, although data on screen-detection measures and interval cancers is routinely collected within organized screening programs, so this may be within the scope of quality assurance evaluations for some programs: institutional requirements will be confirmed by collaborating teams. Hence, each trialist or representative investigator is responsible for ensuring approval by the local/national ethics committee(s) for the purpose of contributing data into this meta-analysis. In addition, ethics approval specific to the proposed IPD meta-analysis has been received from the University of Sydney Ethics Committee for the proposed analyses of IPD sourced from existing studies (Project no.: 2017/143).
The contributing trials remain the custodian of their individual trial data at all times, as well as any additional data provided for the comparison cohorts. All data contributed as part of the IPD meta-analysis agreement will only be used for the purpose of the IPD meta-analysis, and any resulting reports will be reviewed and approved by the collaborating investigators inclusive of representative trialists from each study. Given the moderate scope of the project, the collaborating team includes both the trialists and the investigators responsible for data management. A publication and authorship policy will be developed by the collaborating team, with the guidance of the core governance sub-group, with consideration of standards for ethical authorship practice. Regular (at minimum annual) collaborators’ meetings will be held and formally documented.
Governance sub-group
A core group of investigators will be formed to provide governance and advice on issues relating to project management, data access, publication policy, and to oversee resolution of disagreement or conflicts that may potentially arise amongst collaborating members and teams. It is envisaged that this will include the project lead, a representative of the data management group, and a representative from each trial; a breast cancer consumer representative (breast cancer advocate) will also be invited into this group.
Publication of results
We anticipate that the findings from this work will be presented in a minimum of two separate publications reflecting the two stages of analysis outlined under proposed analyses, and supplemented by presentations in scientific meetings. Results will be presented to the group of collaborators as a draft report circulated for discussion and comment, with subsequent preparation of a revised manuscript for final comment and approval prior to submission.
Discussion
The proposed research as outlined in this protocol is a means of informing researchers and potential collaborators of this work which has been initiated at submission of the manuscript. Finalization of data collection procedures and analysis plans will be complete by the end of 2017. Data collection will occur from late 2017 to late 2018 (screen-detection measures: cancer detection and recall data) and from mid-2018 to mid-2019 (interval cancer data). It is anticipated that results of detection measures should be available by 2019, and that interval cancer results may be available in 2020 (with expected publication 2020–2021).
In the event that studies that meet eligibility criteria decline or are unable to contribute, then we will report this information in any subsequent publication and will provide study-level results (from published aggregate-level data) for each study that did not contribute, to assist in interpreting the extent that this may have biased our work, in line with methods for IPD meta-analysis (15). Our proposal will not collect data on the costs associated with tomosynthesis technology however our results may be used to inform health economics evaluation.
In this protocol, we have defined study eligibility in the context of biennial screening; this screening interval criterion was chosen to ensure that any potential effect of 3D-mammography on interval cancer rates is not removed or diluted through frequent (annual) screening, and to be commensurate with practice in organized population-based breast screening programs. Because the majority of interval breast cancers occur in the second year in biennial screening practice (14), we expect that a measurable impact on interval cancer rates from 3D-mammography screening will be observed in biennial screening studies. This means that our findings will be relevant to population-based breast cancer screening programs that provide biennial screening, but may not generalize to annual screening practice.
By addressing the critical evidence gap on whether 3D-mammography screening reduces interval cancer rates (compared to standard 2D-mammography), we expect that our findings will inform timely translation of 3D-mammography technology into breast screening practice in population-based health programs.
Acknowledgments
Funding: This work was supported by a National Breast Cancer Foundation (Australia), Breast Cancer Research Leadership Fellowship (to N Houssami).
Footnote
Conflicts of Interest: Several authors on this manuscript are investigators of the individual trials to be included in this meta-analysis. These include STORM [N Houssami, D Bernardi], Oslo trial [P Skaane, S Hofvind], MBTST [K Lång, S Zackrisson], STORM 2 [D Bernardi, N Houssami]. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol for analysis of secondary data from published studies has been approved by the University of Sydney Ethics Committee (Project no.: 2017/143).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013;14:583-9. [Crossref] [PubMed]
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014;311:2499-507. [Crossref] [PubMed]
- Houssami N, Macaskill P, Bernardi D, et al. Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading – Evidence to guide future screening strategies. Eur J Cancer 2014;50:1799-807. [Crossref] [PubMed]
- Houssami N, Skaane P. Overview of the evidence on digital breast tomosynthesis in breast cancer detection. Breast 2013;22:101-8. [Crossref] [PubMed]
- Skaane P, Bandos AI, Gullien R, et al. Prospective trial comparing full-field digital mammography (FFDM) versus combined FFDM and tomosynthesis in a population-based screening programme using independent double reading with arbitration. Eur Radiol 2013;23:2061-71. [Crossref] [PubMed]
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013;267:47-56. [Crossref] [PubMed]
- Bernardi D, Macaskill P, Pellegrini M, et al. Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): a population-based prospective study. Lancet Oncol 2016;17:1105-13. [Crossref] [PubMed]
- Lång K, Andersson I, Rosso A, et al. Performance of one-view breast tomosynthesis as a stand-alone breast cancer screening modality: results from the Malmö Breast Tomosynthesis Screening Trial, a population-based study. Eur Radiol 2016;26:184-90. [Crossref] [PubMed]
- Durand MA, Haas BM, Yao X, et al. Early clinical experience with digital breast tomosynthesis for screening mammography. Radiology 2015;274:85-92. [Crossref] [PubMed]
- Greenberg JS, Javitt MC, Katzen J, et al. Clinical performance metrics of 3D digital breast tomosynthesis compared with 2D digital mammography for breast cancer screening in community practice. AJR Am J Roentgenol 2014;203:687-93. [Crossref] [PubMed]
- Haas BM, Kalra V, Geisel J, et al. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology 2013;269:694-700. [Crossref] [PubMed]
- Lourenco AP, Barry-Brooks M, Baird GL, et al. Changes in recall type and patient treatment following implementation of screening digital breast tomosynthesis. Radiology 2015;274:337-42. [Crossref] [PubMed]
- McCarthy AM, Kontos D, Synnestvedt M, et al. Screening outcomes following implementation of digital breast tomosynthesis in a general-population screening program. J Natl Cancer Inst 2014;106:dju316 [Crossref] [PubMed]
- Houssami N, Hunter K. The epidemiology, radiology and biological characteristics of interval breast cancers in population mammography screening. NPJ Breast Cancer 2017;3:12. [Crossref] [PubMed]
- Houssami N, Turner R, Macaskill P, et al. An individual person data meta-analysis of preoperative MRI and breast cancer recurrence. J Clin Oncol 2014;32:392-401. [Crossref] [PubMed]



