Comparison of portal vein embolization, portal vein ligation, associating liver partition and portal vein ligation for staged hepatectomy in cases with a small future liver remnant: a network meta-analysis
Introduction
R0 resection of liver tumour is still the most effective treatment to improve long-term survival (1). However, many patients cannot reach R0 resection because of large tumor size and insufficient future liver remnant (FLR) after major hepatectomy. To completely remove the tumor and ensure sufficient liver remnant, portal vein occlusion, including portal vein embolization (PVE) and portal vein ligation (PVL) (2,3), was introduced. The mechanism of PVE or PVL is to blocking the ipsilateral liver segments’ portal vein blood flow and stimulate contralateral liver growth (4). Using either PVE or PVL to promote residual liver growth is considered as the standard strategy in the cases with primarily unresectable liver malignancies and small FLR (5-7).
Findings regarding the comparative efficacy of PVL and PVE have varied between studies. Some studies demonstrated that PVL was less effective than PVE (8); however, other research indicated that PVL and PVE were equally effective in stimulating growth of the FLR (9). Although the use of PVL or PVE has improved the outcome for some advanced liver cancer patients, the complex blood flow in the liver itself makes it hard to maintain an adequate liver remnant, which would substantially improve survival.
A novel strategy of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) to resect liver tumors in cases with a small liver remnant was introduced in 2007 (10). This special type of hepatectomy is performed in two stages. During the first stage, surgeons conduct the PVL and liver parenchymal transection, which relatively reduces the blood flow of the liver segment containing the tumors and promotes rapid growth of residual liver. Although it has been widely reported that ALPPS induces more effective stimulation of residual liver growth (9,11), the use of ALPPS is highly controversial due to its relatively high morbidity and perioperative mortality (5). However, surgery for liver cancer becomes much safer with the increasing experience of surgeons and improved surgical techniques. Alvarez et al. showed that ALPPS can achieve a high resection completion rate and FLR hypertrophy rate, with a relatively low morbidity and mortality (12). For advanced liver cancer patients, it is difficult for doctors to decide whether PVE, PVL, or ALPPS is the best strategy.
This article aimed to compare the feasibility, safety, and efficacy of PVE, PVL, and ALPPS. The outcome measures included resection completion rate, FLR hypertrophy rate, morbidity, perioperative mortality, and waiting time from first intervention to removal of tumors.
Methods
Search strategy and selection criteria
We conducted a systematic search in the EMBASE, Medline and Cochrane databases for articles published from database inception until April 2016 using the following terms: ALPPS, portal vein ligation, portal vein embolization, staged hepatectomy, and liver resection. There were no language restrictions.
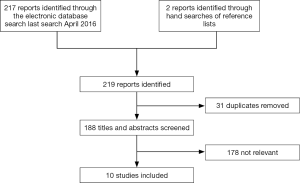
Studies comparing any two of the three techniques (ALPPS, PVL, and PVE) were included in this review. The following articles were excluded: studies including only one or all three of the abovementioned techniques, letters, editorials, opinion articles, conference abstracts, and case reports (Figure 1).
Relevant articles in reference lists were reviewed and duplicate articles were excluded. Two reviewers independently screening the titles and abstracts of each study. Disagreements were resolved by discussion.
Data extraction and quality assessment
Two reviewers independently extracted data. Extracted data included percentage increase in FLR, hepatic resection completion rate, overall morbidity and mortality, and waiting time from first intervention to removal of tumors. a third author was assigned to make sure the accuracy of the data. We conducted a quality assessment for each study using the Newcastle–Ottawa Scale.
Statistical methods
The outcomes we evaluated were waiting time from first intervention to removal of tumors, resection completion rate, rate of FLR increase, postoperative complications, and mortality rate.
Network meta-analysis (NMA) (13) was used to meta-analyze more than two treatments simultaneously. We drew a map of the network that shows which treatments were directly compared with other treatments, and how much information was provided for each treatment and its comparison. We conducted network ranking to evaluate the best operation in terms of waiting time from first intervention to removal of tumors, resection completion rate, FLR increase rate, postoperative complications, and mortality rate using Stata 12 software (StataCorp, College Station, TX, USA). We fitted the sidesplitting model to assess whether the direct overall results and indirect results were consistent. Differences were considered significant if the P value for Z was less than 0.05. We also summarized the direct and indirect results using a consistency or inconsistency model (14-16). Network forests were also provided the forest plot for pairwise meta-analyses. All data was provided as the odds ratio (OR) or mean difference (MD) with confidence interval (CI).
Results
Search results
Our initial electronic search yielded 217 references; of these, eight studies were included according to our inclusion and exclusion criteria. From the reference lists of these eight studies, we identified 2 relevant references for evaluation. A final total of 10 single studies (5,8,9,17-23) and total 451 patients were included in this NMA. The detailed search strategy and the process of study selection are summarized in Figure 1.
Study characteristics
All of the included studies were retrospective. Seven studies (9,17-21) compared PVE and PVL; totally including 218 patients. Three studies (5,22,23) compared PVE with ALPPS; these studies included 259 patients in all. This ten articles were evaluated by Newcastle–Ottawa Scale. Nine studies provided data on resection completion rates, six studies provided data on FLR increase, and seven studies provided waiting time, morbidity, and perioperative mortality data (Table 1).

Full table
Percentage increase in future liver remnant
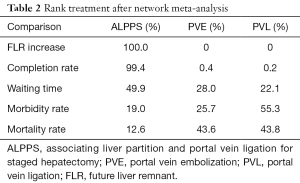
Six studies were analyzed, The overall results indicated a 42.2% reduction in the percentage increase of FLR for PVE compared with ALPPS (95% CI, 26.52–57.87; P<0.001). There was also a 47.65% reduction in the percentage increase of FLR for PVL compared with ALPPS (95% CI, 29.43–65.88; P<0.001). as the forest plots showed in Figure 2A. The network rank analysis showed that the respective probabilities of ALPPS, PVE, and PVL being the best treatment strategy were 100%, 0%, and 0% (Table 2).

Full table
Resection completion rates
Nine studies were analyzed. The results indicated an 89.2% reduction in resection completion rate of hepatic resection for PVE compared with ALPPS (OR =0.108; 95% CI, 0.02–0.585; P=0.01). There was also a 91.6% reduction in resection completion rate for PVL compared with ALPPS (OR =0.084; 95% CI, 0.013–0.532; P=0.009), as the forest plots showed in Figure 2B. The respective probabilities of ALPPS, PVE, and PVL being the best treatment strategy were 99.4%, 0.4% and 0.2% (Table 2).
Perioperative mortality
Seven studies were analyzed. There was a 44.7% reduction in mortality for PVE versus ALPPS; however, this difference was not statistically significant (OR =0.553; 95% CI, 0.158–1.938; P=0.371). A 42.9% non-significant reduction in mortality was also observed for PVL versus ALPPS (OR =0.571; 95% CI, 0.07–4.683; P=0.602), as the forest plots showed in Figure 2C. The respective probabilities of ALPPS, PVE, and PVL being the best treatment strategy were 12.6%, 43.6% and 43.8% (Table 2).
Perioperative morbidity
Seven studies were included in this analysis. Perioperative morbidity including all of the complications from the first step to the end. The pooled results showed a 27.7% reduction in the perioperative morbidity for PVE compared with ALPPS; however, this difference was not statistically significant (OR =0.723; 95% CI, 0.258–2.207; P=0.537), as the forest plots showed in Figure 2D. PVL also had a non-significantly lower morbidity rate, with a 35.6% reduction compared with ALPPS (OR =0.644; 95% CI, 0.166–2.499; P=0.524), as the forest plots showed in Figure 2D. ALPPS, PVE, and PVL had respective probabilities of 19%, 25.7% and 55.3% of being the best treatment strategy (Table 2).
Waiting time between the two stages
Seven studies were included in this analysis. There was an extra 3.42 days wait between the two stages for PVE versus ALPPS; however, this difference was not statistically significant (95% CI, −29.5–36.33; P=0.839). The waiting time for PVL was 7.95 days longer than for ALPPS; although this difference was not statistically significant (95% CI, −35.51–51.4; P=0.72), as the forest plots showed in Figure 2E. ALPPS had a 49.9% probability of being the best treatment strategy, followed by PVE and PVL with respective probabilities of 28% and 22.1% (Table 2).
Discussion
Radical resection is still the best treatment for primary or secondary liver malignancy. Safe hepatectomy mainly concerns the future liver remnant rather than the liver being resected. Patients with huge or multiple tumors undergoing a major hepatectomy may have a small FLR, which can lead to postoperative liver dysfunction or liver failure (24,25). Several strategies have been attempted to improve the surgical resection rates for patients with a small FLR, including PVE, PVL, and ALPPS.
Liver volume partially reflects liver function; the volume calculated using abdominal computed tomography can be used to predict postoperative liver function. A small FLR is defined as FLR ≤ 20% total liver volume (TLV) in normal liver, and FLR ≤ 40% TLV in fibrotic or cirrhotic liver (26). However, remnant liver volume (RLV) to bodyweight ratio (RLV-BWR) is more specific than FLR-TLV as an indicator of the future remnant liver function after extended liver resection. If RLV ≤0.5% of bodyweight, patients are highly likely to experience hepatic dysfunction and postoperative mortality; the incidence of these complications would be even higher in cases involving cirrhotic liver (27).
PVE was introduced in the 1980s to stimulate growth of the remaining portion of the liver. Although PVE is a mature technique that is accepted worldwide, it still has some substantial disadvantages such as percutaneous puncture procedure-related complications, relatively long interval between PVE and the resection operation, and accelerated tumor progression during this interval (28,29); tumor growth is considered the main disadvantage of PVE (30,31).
PVL is widely used in patients with multiple liver metastases from colorectal tumors (32). PVL can be simultaneously performed with the resection of the primary tumor. There are few complications associated with PVL, but some patients undergoing PVE or PVL may fail to have the liver resection performed because of insufficient FLR hypertrophy or disease progression (33,34).
ALPPS was introduced as an alternative to conventional PVE or PVL recent years. Initial experiences indicated that the complication rate and perioperative mortality following ALPPS were superior to those following PVE and PVL; with ALPPS, the FLR can be rapidly increased in a short time (35-38), and the short waiting time from first intervention to removal of tumors can significantly decrease the incidence of tumor progression. ALPPS approach can also be used as a salvage method for patients with inadequate FLR hypertrophy after PVE or PVL (33). PVE, PVL cannot be applied, particularly in cases where the PV is occluded by the tumor (39).
Staged liver resection has been mostly applied in patients with colorectal liver metastases (CLM) since the first time been proposed. Many of the patients accepted preoperative chemotherapy, which may have impacts on the two-stage hepatectomy. But there is study shows that staged liver resection with preoperative chemotherapy almost have the same morbidity and survival benefits as one-stage hepatectomy (40).
Our results showed that ALPPS was the most efficient strategy in promoting the hypertrophy of FLR in the shortest time, and also had the highest resection completion rate among the three surgical strategies; hence, the risk of tumor growth in the interval from first intervention to tumor resection was decreased. Although the mortality and morbidity rate of ALPPS tended to be higher than PVE and PVL, this difference was not statistically significant. Therefore, our study supported previous findings that ALPPS increased the advanced liver cancer resection rate, but did not induce superior morbidity and mortality rates in comparison with PVE and PVL. PVE and PVL had similar efficacy and safety as assessed by many variables. Furthermore, with the progression of surgical techniques, totally laparoscopic ALPPS procedure was performed on cases with bilateral CLM even cirrhotic hepatocellular carcinoma (41-43). This procedure aimed to avoiding adhesions in the first surgery and reducing the rate of complication. Although only a few cases be reported by now, it’s a promising change.
This NMA had some limitations. There were a small number of included studies, and the included studies were retrospective. Furthermore, due to lack of long-term follow-up data, the impact of PVE, PVL, and ALPPS on recurrence and survival cannot be evaluated. All of the included studies cannot avoid bias caused by the lack of a uniform standardized indication for staged liver resection. More randomized clinical trials should be performed to verify the efficacy and safety of PVE, PVL, and ALPPS.
Conclusions
ALPPS was significantly more efficient than PVE and PVL regarding promotion of FLR hypertrophy, resection completion, and waiting time. The three techniques were similar regarding morbidity and mortality.
Acknowledgments
We thank company of Liwen editor for their help in English language revision of this manuscript.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.08.21). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Agrawal S, Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg 2011;253:656-665. [Crossref] [PubMed]
- Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990;107:521-7. [PubMed]
- Honjo I, Suzuki T, Ozawa K, et al. Ligation of a branch of the portal vein for carcinoma of the liver. Am J Surg 1975;130:296-302. [Crossref] [PubMed]
- Corrêa D, Schwartz L, Jarnagin WR, et al. Kinetics of liver volume changes in the first year after portal vein embolization. Arch Surg 2010;145:351-4; discussion 354-5.
- Shindoh J, Vauthey JN, Zimmitti G, et al. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg 2013;217:126-133. [Crossref] [PubMed]
- Mise Y, Sakamoto Y, Ishizawa T, et al. A worldwide survey of the current daily practice in liver surgery. Liver Cancer 2013;2:55-66. [Crossref] [PubMed]
- Shindoh J, Truty MJ, Aloia TA, et al. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg 2013;216:201-9. [Crossref] [PubMed]
- Broering DC, Hillert C, Krupski G, et al. Portal vein embolization vs. portal vein ligation for induction of hypertrophy of the future liver remnant. J Gastrointest Surg 2002;6:905-13. [Crossref] [PubMed]
- Aussilhou B, Lesurtel M, Sauvanet A, et al. Right portal vein ligation is as efficient as portal vein embolization to induce hypertrophy of the left liver remnant. J Gastrointest Surg 2008;12:297-303. [Crossref] [PubMed]
- de Santibañes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the "ALPPS" approach. Ann Surg 2012;255:415-7. [Crossref] [PubMed]
- van Lienden KP, van den Esschert JW, de Graaf W, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 2013;36:25-34. [Crossref] [PubMed]
- Alvarez FA, Ardiles V, de Santibanes M, et al. Associating liver partition and portal vein ligation for staged hepatectomy offers high oncological feasibility with adequate patient safety: a prospective study at a single center. Ann Surg 2015;261:723-32. [Crossref] [PubMed]
- White IR. Multivariate random-effects meta-analysis. Stata Journal 2009;9:40-56.
- Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932-44. [Crossref] [PubMed]
- White IR, Barrett JK, Jackson D, et al. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 2012;3:111-25. [Crossref] [PubMed]
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc 2006;101:447-59. [Crossref]
- van Lienden KP, Hoekstra LT, Bennink RJ, et al. Intrahepatic left to right portoportal venous collateral vascular formation in patients undergoing right portal vein ligation. Cardiovasc Intervent Radiol 2013;36:1572-9. [Crossref] [PubMed]
- Robles R, Marin C, Lopez-Conesa A, et al. Comparative study of right portal vein ligation versus embolisation for induction of hypertrophy in two-stage hepatectomy for multiple bilateral colorectal liver metastases. Eur J Surg Oncol 2012;38:586-93. [Crossref] [PubMed]
- Sturesson C, Keussen I, Tranberg KG. Prolonged chemotherapy impairs liver regeneration after portal vein occlusion - an audit of 26 patients. Eur J Surg Oncol 2010;36:358-64. [Crossref] [PubMed]
- Iida H, Aihara T, Ikuta S, et al. Comparison of percutaneous transhepatic portal vein embolization and unilateral portal vein ligation. World J Gastroenterol 2012;18:2371-6. [Crossref] [PubMed]
- Capussotti L, Muratore A, Baracchi F, et al. Portal vein ligation as an efficient method of increasing the future liver remnant volume in the surgical treatment of colorectal metastases. Arch Surg 2008;143:978-82. [Crossref] [PubMed]
- Knoefel WT, Gabor I, Rehders A, et al. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection. Br J Surg 2013;100:388-94. [Crossref] [PubMed]
- Croome KP, Hernandez-Alejandro R, Parker M, et al. Is the liver kinetic growth rate in ALPPS unprecedented when compared with PVE and living donor liver transplant? A multicentre analysis. HPB 2015;17:477-84. [Crossref] [PubMed]
- Hallet J, Karanicolas PJ, Zih FS, et al. Hypophosphatemia and recovery of post-hepatectomy liver insufficiency. Hepatobiliary Surg Nutr 2016;5:217-24. [Crossref] [PubMed]
- Cieslak KP, Bennink RJ, van Gulik TM. Prediction of postoperative liver failure in patients diagnosed with HCC using 99m Tc-GSA SPECT/CT. Hepatobiliary Surg Nutr 2015;4:203-5. [PubMed]
- Vauthey JN, Dixon E, Abdalla EK, et al. Pretreatment assessment of hepatocellular carcinoma expert consensus statement. HPB (Oxford) 2010;12:289-99. [Crossref] [PubMed]
- Truant S, Olivier O, Geraldine S, et al. Remnant liver volume to body weight ratio≥0.5%: a new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg 2007;204:22-33. [Crossref] [PubMed]
- van Gulik TM, van den Esschert JW, de Graaf W, et al. Controversies in the use of portal vein embolization. Dig Surg 2008;25:436-44. [Crossref] [PubMed]
- Abulkhir A, Limongelli P, Healey AJ, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg 2008;247:49-57. [Crossref] [PubMed]
- Madoff DC, Abdalla EK, Vauthey JN. Portal vein embolization in preparation for major hepatic resection: evolution of a new standard of care. J Vasc Interv Radiol 2005;16:779-90. [Crossref] [PubMed]
- May BJ, Talenfeld AD, Madoff DC. Update on portal vein embolization: evidence-based outcomes, controversies, and novel strategies. J Vasc Interv Radiol 2013;24:241-54. [Crossref] [PubMed]
- Kianmanesh R, Farges O, Abdalla EK, et al. Right portal vein ligation: a new planned two-step all-surgical approach for complete resection of primary gastrointestinal tumors with multiple bilateral liver metastases. J Am Coll Surg 2003;197:164-70. [Crossref] [PubMed]
- Tschuor C, Croome KP, Sergeant G, et al. Salvage parenchymal liver transection for patients with insufficient volume increase after portal vein occlusion -- an extension of the ALPPS approach. Eur J Surg Oncol 2013;39:1230-5. [Crossref] [PubMed]
- Zhang GQ, Zhang ZW, Lau WY, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): a new strategy to increase resectability in liver surgery. Int J Surg 2014;12:437-41. [Crossref] [PubMed]
- Alvarez FA, Ardiles V, Sanchez CR, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): tips and tricks. J Gastrointest Surg 2013;17:814-21. [Crossref] [PubMed]
- Vennarecci G, Laurenzi A, Levi Sandri GB, et al. The ALPPS procedure for hepatocellular carcinoma. Eur J Surg Oncol 2014;40:982-8. [Crossref] [PubMed]
- Jiao LR, Hakim DN, Gall TM, et al. A totally laparoscopic associating liver partition and portal vein ligation for staged hepatectomy assisted with radiofrequency (radiofrequency assisted liver partition with portal vein ligation) for staged liver resection. Hepatobiliary Surg Nutr 2016;5:382-7. [Crossref] [PubMed]
- Alvarez FA, Ardiles V, de Santibanes M, et al. Associating liver partition and portal vein ligation for staged hepatectomy offers high oncological feasibility with adequate patient safety: a prospective study at a single center. Ann Surg 2015;261:723-32. [Crossref] [PubMed]
- Vennarecci G, Laurenzi A, Santoro R, et al. The ALPPS procedure: a surgical option for hepatocellular carcinoma with major vascular invasion. World J Surg 2014;38:1498-503. [Crossref] [PubMed]
- Chun YS, Vauthey JN, Ribero D, et al. Systemic chemotherapy and two-stage hepatectomy for extensive bilateral colorectal liver metastases: perioperative safety and survival. J Gastrointest Surg 2007;11:1498-504. [Crossref] [PubMed]
- Machado MA, Makdissi FF, Surjan RC. Totally laparoscopic ALPPS is feasible and may be worthwhile. Ann Surg 2012;256:e13-e19. [Crossref] [PubMed]
- Xiao L, Li JW, Zheng SG. Totally laparoscopic ALPPS in the treatment of cirrhotic hepatocellular carcinoma. Surg Endosc 2015;29:2800-1. [Crossref] [PubMed]
- Schelotto PB, Gondolesi G. Laparoscopy in ALPPS Procedure: When We Can Do It? Ann Surg 2017;265:e30-e31. [Crossref] [PubMed]