
Gold nanoparticles in radiation research: potential applications for imaging and radiosensitization
Applications of radiation in cancer imaging and treatment
Both ionizing radiation therapy and CT-based imaging modalities are mainstays of cancer treatment and diagnosis. These techniques, lifesaving as they are, have potential side effects and limitations; thus, adjuvants and complementary agents would be a welcome addition. From a therapeutic perspective, despite significant advances in technology, radiation therapy does not always achieve local control of the primary tumor, while at the same time potentially causing normal tissue toxicity. Radiosensitizing adjuvants that enhance the dose specifically absorbed by tumor tissue can result in enhanced tumor killing for any given total radiation dose compared to radiation therapy alone. From an imaging perspective, traditional iodine-based contrast agents are often limited by fast clearance, short imaging times, requirement for high doses of radiation, and insufficient contrast resolution (1). An agent with enhanced X-ray attenuation capabilities could potentially improve sensitivity and resolution of tumor imaging, while exposing patients to lower radiation doses. Gold nanoparticles (GNPs) are currently being studied in both of these therapeutic and diagnostic roles, and have thus far shown great potential clinical application.
Properties and functionalization of GNPs
Physicochemical properties of GNPs
The anti-cancer potential of GNPs stems from several advantageous physicochemical properties (Figure 1). First, numerous studies have established gold’s safety and biocompatibility both in vitro and in vivo (2-6), suggesting that GNPs can be safely administered with minimal inflammatory activation (6) and few local or systemic side effects. Second, gold can be easily manufactured in a variety of shapes and sizes, and possesses easily controllable surface chemistry allowing functionalization with various biologically useful molecules to help evade immune detection and improve stability, tumor-targeting, and crossing of biophysical barriers such as the blood-brain barrier (7,8). Third, gold’s high atomic number (Au, 79) allows high absorption and enhancement of ionizing radiation, as well as superior X-ray attenuation for imaging applications. Other physical characteristics of gold such as surface plasmon resonance and Raman scattering activity (9) have been exploited in non-radiation based cancer applications including optical imaging and photoacoustic tomography of tumors, drug delivery vehicles, tumor-specific photothermal therapy agents, antiangiogenic agents, and molecular reporters (10). In this review, we will focus on radiation-based therapeutic and diagnostic applications of GNPs.
GNP production, functionalization, and delivery to tumor tissue
Gold nanoparticles can be easily produced in uniform sizes and shapes, including nanospheres, nanorods, shells, and cages (10). Classic methods of gold nanosphere synthesis include citrate reduction of aqueous HAuCl4 by the Turkevich method (11); and the Brust-Schiffrin two-phase synthesis method which uses NaBH4 as a reducing agent and a mercapto-containing binding agent (12). In both methods, nanosphere size can be tuned by altering the ratio of gold to reducing substance. Other reductants have been employed to improve GNP yield and tunability; while surface ligands such as tumor-targeting antibodies, as discussed below, have been employed to modify GNP functionality and delivery.
Biological molecules such as DNA and RNA are also capable of being functionalized with GNPs. There are several ways to achieve this, including functionalization that takes advantage of the electrostatic interactions between GNPs and the target biological molecule to create GNP bioconjugates. For example, positively charged GNPs can bind through stable ionic interactions to negatively charged and nucleophilic moieties, i.e., GNPs may interact with the phosphate ester backbone of nucleic acids within DNA and RNA (13).
Targeted delivery of GNPs to tumor tissue can be accomplished in a variety of ways. Direct routes of intratumoral injection and intraperitoneal administration have been described for targeting of lung cancers (14). More clinically relevant, intravenously (IV) administered bare gold nanoparticles exhibit selective accumulation in tumor tissue due to the tumor’s characteristic leaky fenestrated vasculature and impaired lymphatic clearance—the enhanced permeability and retention (EPR) effect (15,16). For example, Hainfeld et al. found that a one-time injection (2.7 g Au/kg body weight) of 1.9 nm GNPs led to accumulation within tumors of up 7 mg Au/g, for a selective tumor-to-normal-tissue gold concentration ratio of 8:1 (17).
The EPR-dependent passive accumulation strategy for bare GNP delivery is limited, however, by the inherent heterogeneities of tumor vasculature, especially in necrotic poorly-vascularized areas of tumor. In addition, rapid renal clearance, opsonization, and nonspecific phagocytosis of nanoparticles by the reticuloendothelial system (RES) pose a challenge to delivery and persistence of adequate nanoparticle concentrations in the target site (7). Moreover, high interstitial pressure within tumors may also represent a barrier to the EPR effect as has been described elsewhere (18).
Various ligands and GNP surface modifications have been employed to address these limitations. Coating GNPs with polyethylene glycol (PEG), for instance, improves stability and persistence in circulation, allowing greater accumulation in tumor tissue and providing a hydrophobic barrier to RES phagocytosis and uptake (19). Work in our labs has shown that intravenously injected PEG-coated GNPs can accumulate in mouse sarcoma flank tumors to concentrations 10 times that of muscle and 50 times that of brain (data not shown).
More specific tumor-targeting can be achieved by surface conjugation of antibodies to markers overexpressed in tumors, such as EGF, HER2, and folate (20-23). Generally, functionalization of gold is accomplished either by direct thiol-modification of the targeting ligand or through the attachment of a targeting ligand to GNPs that have been modified within a coating material (e.g., polymer, lipid, etc.). Marega et al. used a plasma-polymerized allylamine coating to allow bioconjugation of tumor-targeting EGFR monoclonal antibodies to GNPs (20). Folic acid-conjugated GNPs have been produced by grafting on a PEG polymer chain with thioctic acid and folic acid on opposite ends (21); and Hainfeld et al. produced Her2-targeted GNPs by coating 15 nm GNPs with PEG and covalently coupling them to anti-Her2 antibodies (23). Other non-tumor targeting ligands have also been employed to broaden GNP functionality—for instance, Kumar et al. equipped ultra-small GNPs with both a therapeutic peptide (PMI/p12) and also a targeting peptide (neuropilin-1), which provided regulated membrane receptor-mediated cellular internalization (24). Furthermore, attempts have also been made to target GNPs to tumors by exploiting the unique tumor microenvironment, which may include matrix metalloprotease (MMP) expression, low pH, and elevated glucose metabolism. For example, Ayogan et al. have initially characterized 2-deoxy-D-glucose (2DG)-labeled GNPs for potential cancer imaging (25).
Indeed, most targeting ligands specific for overexpressed membrane receptors also have the advantage of increased receptor-mediated internalization into tumor cells (26)—an improvement upon the nonspecific cellular uptake of bare GNPs (27). The rates of internalization are also highly dependent on size and physical dimensions of nanoparticles (27). The tuning of geometry, surface modification, and tumor-targeting functionality of GNPs are under active investigation and can be accomplished in a variety of ways. Once delivered to tumor tissue, GNPs can be leveraged as multifunctional radiotherapy and imaging adjuvants, as will be discussed below.
Radiosensitization
Numerous studies have shown that gold nanoparticles delivered to tumor tissue can selectively enhance radiation therapy efficacy leading to differentially increased tumor cell killing. Though the exact mechanisms are unclear, radiosensitization is generally attributed to increased photon absorption of high-Z elements, and the resulting transfer of a larger portion of primary ionizing photon energy to tumor tissue (28). Theoretical dose enhancement achieved by gold radiosensitization as predicted by Monte Carlo studies is significant (up to 200% or more) (29,30).
The photoelectric mechanism of radiosensitization predominantly occurs at kilovoltage (kV) energies which are generally less clinically relevant (with the exception of brachytherapy); however, studies have shown dose enhancement and radiosensitization at megavoltage (MV) energies as well (5,31,32). Jain et al. showed cell-specific radiosensitization in breast cancer MDA-MB-231 cells, with comparable dose-enhancing ratios at kV and MV energies (32). Berbeco et al. suggest that GNPs can enhance the tumor-killing efficacy of 6 MV X-rays by boosting radiation dose to the tumor microvasculature and endothelial cells (31). In vitro work by Chitthrani showed that 50 nm GNPs radiosensitize in both lower and higher energy photon ranges, with dose modification factors (DMF) of 1.66 for 105 kVp and 1.17 for 6 MVp (33).
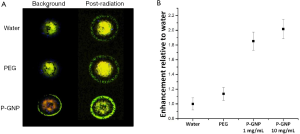
To test our hypothesis that GNP-induced radiosensitization is also present in the MV radiation energies, we conducted preliminary studies utilizing specialized radiochromic film to measure potential MV range energy dose enhancement in the presence of GNPs. The dose enhancement was calculated by subtracting the background dose map before a single exposure to 2 Gy [6 MV beam energy, in the presence of water, PEG vehicle, or PEGylated-GNP (P-GNP) solution on film] from the post-radiation dose map. In a comparison of background and post-radiation dose maps of water, PEG alone, and PEG-GNPs, we preliminarily found that there is negligible enhancement due to the PEG vehicle alone compared to water as would be expected. However, as shown in a representative dose map image, P-GNPs demonstrate significant enhancement of absorbed dose in radiochromic film measurements (Figure 2A). We quantified these results for 2 Gy of radiation delivered by a Varian TrueBeam system in Flattening Filter Free (FFF) mode which shows significant enhancement in the presence of P-GNP compared to water or PEG alone (Figure 2B). Taken together, this preliminarily suggests that GNPs are capable of radiosensitization in the clinically relevant MV range of radiation energies. In terms of mechanism, short-range low-energy Auger electrons which deliver a precise lethal dose in their immediate vicinity (34,35) could help to explain higher-energy radiosensitization. Indeed, Zheng et al. concluded that GNP-induced radiosensitization was largely attributable to the production of low-energy secondary electrons (which are about three times more efficient than X-rays in causing DNA damage), and that this radiosensitizing mechanism operates at MV photon beam energies commonly used in radiotherapy (36).
Alternate biological mechanisms have also been proposed to account for radiosensitization seen at MV energies; beyond serving as an inert photon-absorbing element, gold may also act as a biologically active agent that enhances radiation damage by inducing cellular responses such as cell cycle acceleration (37), cytokinesis arrest, increased apoptosis (5,32), and ROS-induced DNA damage (38). Although in vivo studies of radiosensitization at higher energies are limited, preliminary modeling and cell line results suggest that GNPs can also be effective radiosensitizers in the MV range, with direct applicability to clinical radiation therapy.
Studies in cell line and animal models have shown various degrees of radiosensitization and tumor cell killing. Hainfeld et al. first showed in 2004 that intravenously injected 1.9 nm GNPs accumulated in and enhanced radiation-induced killing of mammary carcinomas in mice, leading to a 1-year survival of 86% compared to 20% with X-rays alone and 0% with gold alone (17). Chang et al. subsequently showed that GNPs accumulate inside melanoma cells and enhance the efficacy of ionizing radiation, inducing tumor cell apoptosis, retarding tumor growth, and resulting in significantly increased survival in tumor-bearing mice (39). Similar GNP radioenhancement has been shown in head and neck squamous cell carcinoma (40), prostate cancer (41), and ovarian cancer (42). Work in our laboratory has shown that glioma cells and even brain tumors, despite their protection from the circulation by the blood-brain barrier, can be targeted and efficiently radiosensitized by PEGylated GNPs, leading to enhanced DNA damage, tumor cell killing (Figure 3), and improved survival (Figure 4).
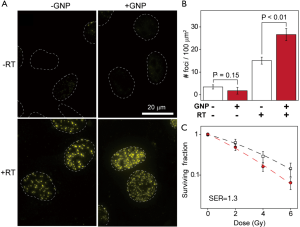
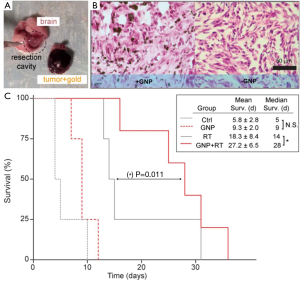
Intriguingly, proton radiotherapy has also been shown to exert increased tumor-killing efficacy when directed against gold-loaded tumors. Polf et al. showed that prostate tumor cells with internalized gold nanoparticles exhibited increased ionization density and a lower surviving fraction when irradiated with proton beams compared with cells exposed to proton therapy alone. They approximate a clinically significant 15-20% increase in the relative biological effectiveness of proton therapy of gold-loaded tumor cells compared to proton therapy in the absence of gold, and attribute this effect to proton-Au scatter interactions and production of low energy delta-ray electrons, which result in lethal intracellular damage and lower cell survival for any given proton dose (43). More recently, Kim et al. used protons (10-41 Gy) to irradiate mouse tumors loaded with gold and iron nanoparticles, and found significant dose enhancement with increased intracellular ROS generation in vitro as well as increased tumor regression and mouse survival in vivo, due to release of secondary electrons and particle-induced radiation (44).
The predominant mechanisms and extent of GNP-induced radiosensitization are likely dependent on a multiple variables, including nanoparticle size and shape (33,45), surface coating (26,46), radiation dose and energy (40), and tumor type. Other GNP-assisted mechanisms, such as hyperthermia and chemosensitization, may also work in synergy with radiosensitization (40,46). Clearly, more studies are needed to optimize GNP surface architecture and elucidate mechanisms behind gold-enhanced tumor cell killing; however, the clinical applicability and therapeutic promise of GNPs as safe and effective adjuvants in radiation therapy for cancer seems increasingly clear.
Imaging
Contrast agents can improve the accuracy of tumor diagnosis, staging, and treatment planning by providing superior definition of tumor volumes and vasculature (47,48). Gold has been demonstrated as an effective experimental X-ray contrast agent that can overcome numerous obstacles of traditional iodine-based contrast agents. At energy ranges used for clinical CT imaging, gold exhibits much higher mass-energy absorption coefficient than iodine (Figure 5); indeed, gold’s higher atomic number and X-ray absorption coefficient results in about 2.7 times greater attenuation per unit weight than iodine, which could translate to better image contrast at lower radiation doses (1). Surface modifications can enhance this effect—Kim et al. found the attenuation coefficient achieved by PEG-coated GNPs to be 5.7 times higher than by current iodine-based CT contrast agents (49). Gold’s physical properties also allow good contrast in higher X-ray photon energies (80-100 keV) which exhibit lower soft tissue absorption and thus lower radiation toxicity to patients (1,50). The higher molecular weight of GNPs, along with its ability to be conjugated to various antibiofouling surface molecules such as PEG, also lends it stability and persistence in circulation, allowing longer imaging times and less renal toxicity (49).
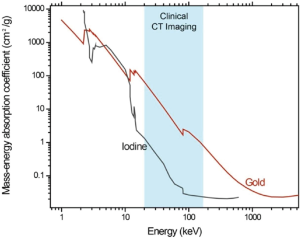
Either by passive EPR-assisted accumulation or targeted delivery, intravenously administered GNPs can localize to tumor tissue and allow CT-assisted visualization of tumor-associated vasculature and borders. After intravenously injecting GNPs into mice implanted with breast tumors, Hainfeld et al. found sufficient CT contrast enhancement enabling direct imaging of GNP-loaded tumors as well as angiogenic and hypervascularized regions (23). Chien et al. found that bare GNPs in conjunction with heparin injection also provided sufficient contrast to allow in vivo detection of tumor microvessels, suggesting their application in tumor-related angiography (51). Surface modifications have also been shown to be useful—Kim et al. demonstrated the use of PEGylated GNPs as long-circulating contrast agents in the imaging of hepatoma (49); and Wang et al. showed that acetylated dendrimer-entrapped GNPs could be used for both in vitro and in vivo CT imaging of adenocarcinoma (14). Work in our laboratories have shown that PEGylated GNPs can serve as long-circulating vascular blood pool CT imaging agents as well as CT contrast agents for sarcoma tumors in mice (Figure 6). Figure 6A shows coronal CT images through a non-tumor bearing mouse before, immediately after and 20 hours post injection of PEGylated GNPs which highlight the long-circulating contrast properties of this agent. Figure 6B demonstrates an axial CT image of well-defined GNP-loaded, contrast-enhancing orthotopic sarcoma flank tumor. CT images can also be reconstructed in the x,y,z coordinates to create a 3-dimensional representation of GNP accumulation within tumors, as shown in Figure 6C, which may be useful in future studies to define the parameters and microenvironmental factors that lead to heterogenous uptake within tumors. One could also speculate that GNPs may have utility in the study of vascular renormalization that may occur with various targeted agents (52).

Gold-based contrast agents may also serve as molecular CT imaging platforms for tumors that are undetectable by structural and anatomical imaging modalities. Popovtzer et al. showed that immuno-targeted gold nanorods coated with tumor-selective antibodies can bind to head and neck squamous cell carcinoma cells, accumulating to concentrations sufficient to provide 5 times greater CT attenuation compared to untargeted cancer cells or normal cells (48). Additionally, this technique has the potential advantage of selectively identifying aggressive tumor cells by specifically targeting antigens overexpressed on cells with metastatic behavior (48). This molecular imaging concept has also been supported in vivo; Reuveni et al. showed that EGF-conjugated GNPs, when intravenously injected into nude mice, efficiently homed to and caused contrast enhancement of head and neck cancers too small to be detectable through conventional CT (53). Furthermore, Eck et al. demonstrated that anti-CD4-targeted GNPs could distinctly enhance the X-ray contrast of peripheral lymph nodes (54) which is directly relevant to the radiation treatment planning of target volumes.
In addition to their applications in CT imaging, GNPs can also be conjugated to paramagnetic elements such as iron and gadolinium to form MRI-active contrast agents. This is important for two reasons: improved sensitivity (the sensitivity of CT imaging of GNPs tends to fall off at a concentration of about 0.5 mg/mL) (23), and the potential acquisition of additional pathological or molecular information with complementary imaging techniques. A gold-iron oxide micellar formulation is currently being investigated in our laboratories as a contrast agent for both CT and MRI imaging of tumors in mice. Choi et al. demonstrated the use of hybrid FePt-Au nanoparticles in molecular MR imaging and other biological detection modalities (55); and dumbbell-shaped Au-Fe3O4 nanoparticles have been reported as simultaneous optical and MR imaging agents (56). Similarly, Kim et al. showed dual-modality CT and MRI blood pool imaging using GNPs coated with Gadolinium-chelate (57). Due to its versatility and ability to be conjugated to other elements, gold may be incorporated into versatile imaging nanoplatforms capable of multimodal diagnostic applications.
Conclusions: limitations and theranostic possibilities
GNP safety
Gold has a long history of use in medical practice and continues today as treatment for conditions such as rheumatoid arthritis (58). Although bulk gold is generally accepted to be nontoxic and has been approved for clinical use in some human diseases, nanoparticle formulations of gold carry potentially more risk due to small size and ability to disseminate, penetrate, and persist in organ systems. Smaller nanoparticles have been shown to cause apoptosis, reactive oxygen species, and necrosis of various tissues due to their deeper penetration and wider systemic distribution (6,59,60).
One potential concern with the use of GNPs may be protracted elimination from the liver (61-63)—with one study reporting only 9% decrease in the content of gold in the liver from day 1 to 6 months following the intravenous injection of 40 nm GNPs (64); and another study showing inflammatory and apoptotic changes in liver tissue after injection of 13 nm PEG-GNPs (65). Nephrotoxicity is also a potential risk of GNP administration, with gold nanoparticles shown to be capable of penetrating renal cells (66) and accumulating in kidney tissue (2). Reassuringly, however, when 12.5 nm GNPs were administered to mice daily for 8 days, no evidence of toxicity was observed in terms of survival, behavior, animal weight, organ morphology, blood biochemistry, and tissue histology over a period of two-plus months (67). In addition, the percent of GNPs uptaken by both liver and kidney decreased with increasing doses, suggesting that GNPs are in fact cleared from the body (4). In vitro studies have also showed that even high relatively GNP concentrations inflict little cytotoxicity on various kidney cell lines (2).
In general, nanoparticles <~6 nm are primarily renally cleared and have low circulation times. Larger particles enjoy a prolonged systemic circulation thus enhancing accumulation within tumors. These particles may remain in the reticuloendothelial system (RES) for long periods of time; however, numerous studies have suggested that larger colloidal GNPs exhibit lower cytotoxicity, possibly due to diminished binding to DNA and other key molecules (2). There is a tradeoff between the larger particle size necessary for molecular imaging (especially for targeted imaging given a limited number of surface receptors) and the effective clearance of smaller particles. This remains a hurdle for their utilization in diagnostic imaging.
Although still not fully understood, GNP persistence and toxicity is governed by factors including cell type as well as GNP functionalization and size. In any case, these concerns have not prevented the use of gold in patients with poor cancer prognoses. In fact, several GNP formulations have already entered clinical trials for cancer treatment, including CYT-6091 (www.clinicaltrials.gov: NCT00356980) and AuroShell® particles (silica core with a gold shell). More safety studies of GNPs in various formulations are needed before further clinical implementation can occur.
Theranostic possibilities
Most studies to date have investigated GNPs in either a radiosensitizing or imaging role; combined theranostic anti-cancer applications of GNPs have mainly focused on non-radiation based drug-delivery and molecular or cellular imaging modalities (68). However, studies investigating GNP-induced radiosensitization have hinted at imaging applications as well—for example, while their primary aim was to demonstrate radiosensitization of mammary carcinoma, Hainfeld et al. also found X-ray contrast enhancement of the gold-loaded tumor (17). Work in our laboratories has also shown that PEGylated-GNPs can function simultaneously as both a CT contrast agent and a radiosensitizer. Future studies are still needed to more fully investigate the multifunctional theranostic potential of GNPs and are currently ongoing.
Conclusions
Gold nanoparticles are novel agents with strong therapeutic and diagnostic potential in a wide variety of cancer applications. These nanoagents possess many attractive physicochemical properties including biocompatibility, easy synthesis and modification, and a high Z coefficient; however, potential safety concerns and mechanisms of radiosensitization at different energy ranges still need to be fully elucidated before clinical implementation. Although their multifunctional potential remains to be fully explored, GNPs could represent an ideal theranostic adjuvant in radiation-based diagnostic and therapeutic anti-cancer modalities. We are currently actively engaged in the studies to move these potential theranostic agents closer to clinical implementation.
Acknowledgments
Funding: This work was supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke (K08 NS076548). J.F.D. was supported on a Burroughs Wellcome Career Award for Medical Scientists (1006792).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Rao V. L. Papineni, Pataje G.S. Prasanna, Mansoor M. Ahmed) for the series “Nanotechnology in Radiation Research” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.08.09). The series “Nanotechnology in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hainfeld JF, Slatkin DN, Focella TM, et al. Gold nanoparticles: a new X-ray contrast agent. Br J Radiol 2006;79:248-53. [PubMed]
- Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev 2011;40:1647-71. [PubMed]
- Connor EE, Mwamuka J, Gole A, et al. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005;1:325-7. [PubMed]
- Lasagna-Reeves C, Gonzalez-Romero D, Barria MA, et al. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem Biophys Res Commun 2010;393:649-55. [PubMed]
- Coulter JA, Jain S, Butterworth KT, et al. Cell type-dependent uptake, localization, and cytotoxicity of 1.9 nm gold nanoparticles. Int J Nanomedicine 2012;7:2673-85. [PubMed]
- Shukla R, Bansal V, Chaudhary M, et al. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir 2005;21:10644-54. [PubMed]
- Jain S, Hirst DG, O’Sullivan JM. Gold nanoparticles as novel agents for cancer therapy. Br J Radiol 2012;85:101-13. [PubMed]
- Praetorius NP, Mandal TK. Engineered nanoparticles in cancer therapy. Recent Pat Drug Deliv Formul 2007;1:37-51. [PubMed]
- Kah JC, Kho KW, Lee CG, et al. Early diagnosis of oral cancer based on the surface plasmon resonance of gold nanoparticles. Int J Nanomedicine 2007;2:785-98. [PubMed]
- Cai W, Li T, Zhong H, et al. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol Sci Appl 2008;17-32.
- Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc 1951;11:55-75.
- Brust M, Walker M, Bethell D, et al. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid-liquid system. J Chem Soc Chem Commun 1994;7:801-2.
- De Long RK, Reynolds CM, Malcolm Y, et al. Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotechnol Sci Appl 2010;8:53-63.
- Wang H, Zheng L, Peng C, et al. Computed tomography imaging of cancer cells using acetylated dendrimer-entrapped gold nanoparticles. Biomaterials 2011;32:2979-88. [PubMed]
- Iyer AK, Khaled G, Fang J, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today 2006;11:812-8. [PubMed]
- Fang J, Sawa T, Maeda H. Factors and mechanism of “EPR” effect and the enhanced antitumor effects of macromolecular drugs including SMANCS. Adv Exp Med Biol 2003;519:29-49. [PubMed]
- Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol 2004;49:N309-15.
- Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J Cell Biol 2010;188:759-68. [PubMed]
- Cho WS, Cho M, Jeong J, et al. Size-dependent tissue kinetics of PEG-coated gold nanoparticles. Toxicol Appl Pharmacol 2010;245:116-23. [PubMed]
- Marega R, Karmani L, Flamant L, et al. Antibody-functionalized polymer-coated gold nanoparticles targeting cancer cells: an in vitro and in vivo study. Journal of Materials Chemistry 2012;22:21305-12.
- Dixit V, Van den Bossche J, Sherman DM, et al. Synthesis and grafting of thioctic acid-PEG-folate conjugates onto Au nanoparticles for selective targeting of folate receptor-positive tumor cells. Bioconjug Chem 2006;17:603-9. [PubMed]
- Zhao X, Li H, Lee RJ. Targeted drug delivery via folate receptors. Expert Opin Drug Deliv 2008;5:309-19. [PubMed]
- Hainfeld JF, O’Connor MJ, Dilmanian FA, et al. Micro-CT enables microlocalisation and quantification of Her2-targeted gold nanoparticles within tumour regions. Br J Radiol 2011;84:526-33. [PubMed]
- Kumar A, Ma H, Zhang X, et al. Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials 2012;33:1180-9. [PubMed]
- Aydogan B, Li J, Rajh T, et al. AuNP-DG: deoxyglucose-labeled gold nanoparticles as X-ray computed tomography contrast agents for cancer imaging. Mol Imaging Biol 2010;12:463-7. [PubMed]
- Kong T, Zeng J, Wang X, et al. Enhancement of radiation cytotoxicity in breast-cancer cells by localized attachment of gold nanoparticles. Small 2008;4:1537-43. [PubMed]
- Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 2006;6:662-8. [PubMed]
- Hainfeld JF, Dilmanian FA, Slatkin DN, et al. Radiotherapy enhancement with gold nanoparticles. J Pharm Pharmacol 2008;60:977-85. [PubMed]
- Hainfeld JF, Dilmanian FA, Slatkin DN, et al. Radiotherapy enhancement with gold nanoparticles. J Pharm Pharmacol 2008;60:977-85. [PubMed]
- Cho SH. Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: a preliminary Monte Carlo study. Phys Med Biol 2005;50:N163-73.
- Berbeco RI, Ngwa W, Makrigiorgos GM. Localized dose enhancement to tumor blood vessel endothelial cells via megavoltage X-rays and targeted gold nanoparticles: new potential for external beam radiotherapy. Int J Radiat Oncol Biol Phys 2011;81:270-6. [PubMed]
- Jain S, Coulter JA, Hounsell AR, et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int J Radiat Oncol Biol Phys 2011;79:531-9. [PubMed]
- Chithrani DB, Jelveh S, Jalali F, et al. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat Res 2010;173:719-28. [PubMed]
- Butterworth KT, McMahon SJ, Currell FJ, et al. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012;4:4830-8. [PubMed]
- Lechtman E, Chattopadhyay N, Cai Z, et al. Implications on clinical scenario of gold nanoparticle radiosensitization in regards to photon energy, nanoparticle size, concentration and location. Phys Med Biol 2011;56:4631-47. [PubMed]
- Zheng Y, Hunting DJ, Ayotte P, et al. Radiosensitization of DNA by gold nanoparticles irradiated with high-energy electrons. Radiat Res 2008;169:19-27. [PubMed]
- Roa W, Zhang X, Guo L, et al. Gold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycle. Nanotechnology 2009;20:375101 [PubMed]
- Butterworth KT, Coulter JA, Jain S, et al. Evaluation of cytotoxicity and radiation enhancement using 1.9 nm gold particles: potential application for cancer therapy. Nanotechnology 2010;21:295101 [PubMed]
- Chang MY, Shiau AL, Chen YH, et al. Increased apoptotic potential and dose-enhancing effect of gold nanoparticles in combination with single-dose clinical electron beams on tumor-bearing mice. Cancer Sci 2008;99:1479-84. [PubMed]
- Hainfeld JF, Dilmanian FA, Zhong Z, et al. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys Med Biol 2010;55:3045-59. [PubMed]
- Zhang X, Xing JZ, Chen J, et al. Enhanced radiation sensitivity in prostate cancer by gold-nanoparticles. Clin Invest Med 2008;31:E160-7. [PubMed]
- Geng F, Song K, Xing JZ, et al. Thio-glucose bound gold nanoparticles enhance radio-cytotoxic targeting of ovarian cancer. Nanotechnology 2011;22:285101 [PubMed]
- Polf JC, Bronk LF, Driessen WH, et al. Enhanced relative biological effectiveness of proton radiotherapy in tumor cells with internalized gold nanoparticles. Appl Phys Lett 2011;98:193702 [PubMed]
- Kim JK, Seo SJ, Kim HT, et al. Enhanced proton treatment in mouse tumors through proton irradiated nanoradiator effects on metallic nanoparticles. Phys Med Biol 2012;57:8309-23. [PubMed]
- Zhang XD, Wu D, Shen X, et al. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012;33:6408-19. [PubMed]
- Hébert EM, Debouttière PJ, Lepage M, et al. Preferential tumour accumulation of gold nanoparticles, visualised by Magnetic Resonance Imaging: radiosensitisation studies in vivo and in vitro. Int J Radiat Biol 2010;86:692-700. [PubMed]
- Essig M, Debus J, Schlemmer HP, et al. Improved tumor contrast and delineation in the stereotactic radiotherapy planning of cerebral gliomas and metastases with contrast media-supported FLAIR imaging. Strahlenther Onkol 2000;176:84-94. [PubMed]
- Popovtzer R, Agrawal A, Kotov NA, et al. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett 2008;8:4593-6. [PubMed]
- Kim D, Park S, Lee JH, et al. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. J Am Chem Soc 2007;129:7661-5. [PubMed]
- Shilo M, Reuveni T, Motiei M, et al. Nanoparticles as computed tomography contrast agents: current status and future perspectives. Nanomedicine (Lond) 2012;7:257-69. [PubMed]
- Chien CC, Chen HH, Lai SF, et al. Gold nanoparticles as high-resolution X-ray imaging contrast agents for the analysis of tumor-related micro-vasculature. J Nanobiotechnology 2012;10:10. [PubMed]
- Cerniglia GJ, Pore N, Tsai JH, et al. Epidermal growth factor receptor inhibition modulates the microenvironment by vascular normalization to improve chemotherapy and radiotherapy efficacy. PLoS One 2009;4:e6539 [PubMed]
- Reuveni T, Motiei M, Romman Z, et al. Targeted gold nanoparticles enable molecular CT imaging of cancer: an in vivo study. Int J Nanomedicine 2011;6:2859-64. [PubMed]
- Eck W, Nicholson AI, Zentgraf H, et al. Anti-CD4-targeted gold nanoparticles induce specific contrast enhancement of peripheral lymph nodes in X-ray computed tomography of live mice. Nano Lett 2010;10:2318-22. [PubMed]
- Choi JS, Jun YW, Yeon SI, et al. Biocompatible heterostructured nanoparticles for multimodal biological detection. J Am Chem Soc 2006;128:15982-3. [PubMed]
- Xu C, Xie J, Ho D, et al. Au-Fe3O4 dumbbell nanoparticles as dual-functional probes. Angew Chem Int Ed Engl 2008;47:173-6. [PubMed]
- Kim HK, Jung HY, Park JA, et al. Gold nanoparticles coated with gadolinium-DTPA-bisamide conjugate of penicillamine (Au@GdL) as a T1-weighted blood pool contrast agent. J Mater Chem 2010;20:5411-7.
- Fricker S. Medical uses of gold compounds: past, present, and future. Gold Bulletin 1996;29:53-60.
- Pan Y, Neuss S, Leifert A, et al. Size-dependent cytotoxicity of gold nanoparticles. Small 2007;3:1941-9. [PubMed]
- Lim ZZ, Li JE, Ng CT, et al. Gold nanoparticles in cancer therapy. Acta Pharmacol Sin 2011;32:983-90. [PubMed]
- Balasubramanian SK, Jittiwat J, Manikandan J, et al. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials 2010;31:2034-42. [PubMed]
- Goel R, Shah N, Visaria R, et al. Biodistribution of TNF-alpha-coated gold nanoparticles in an in vivo model system. Nanomedicine (Lond) 2009;4:401-10. [PubMed]
- James WD, Hirsch LR, West JL, et al. Applications of INAA to the build-up and clearance of gold nanoshells in clinical studies in mice. J Radioanal Nucl Chem 2007;271:455-9.
- Sadauskas E, Danscher G, Stoltenberg M, et al. Protracted elimination of gold nanoparticles from mouse liver. Nanomedicine 2009;5:162-9. [PubMed]
- Cho WS, Cho M, Jeong J, et al. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol Appl Pharmacol 2009;236:16-24. [PubMed]
- Sereemaspun A, Rojanathanes R, Wiwanitkit V. Effect of gold nanoparticle on renal cell: an implication for exposure risk. Ren Fail 2008;30:323-5. [PubMed]
- Lasagna-Reeves C, Gonzalez-Romero D, Barria MA, et al. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem Biophys Res Commun 2010;393:649-55. [PubMed]
- Heo DN, Yang DH, Moon HJ, et al. Gold nanoparticles surface-functionalized with paclitaxel drug and biotin receptor as theranostic agents for cancer therapy. Biomaterials 2012;33:856-66. [PubMed]


