
BCL9L and caspase-2—new guardians against aneuploidy
Every time cell divides, the chromosomes must be duplicated and segregated equally into the two daughter cells. Duplication and segregation of chromosomes is a dynamic process that involves highly orchestrated processes to ensure faithful transfer of the genetic information. Infrequently, errors in mitosis can occur that cause the cells to missegregate one or more chromosomes, which leads usually to irreversible cell cycle arrest or cell death. In rare occasions, some of these cells survive to give rise to daughter cells with either loss or gain of chromosomes; a condition known as “aneuploidy”. In humans, aneuploidy is rarely compatible with life and is recognized as the major cause of spontaneous miscarriages. At the cellular level, depending on the severity of the errors, the propagation of cells after chromosome missegregation is restricted by induction of G1 arrest or apoptosis or by severe proliferation defect of the arising aneuploid progeny.
Intriguingly, aneuploidy is also highly prevalent in cancer. About 80% of solid tumors and approximately 60% of hematopoietic cancers show variable aneuploid karyotypes. Aneuploidy positively correlates with resistance to anti-cancer treatment and poor prognosis (1). The frequent occurrence of aneuploidy and the ongoing missegregation—so called chromosomal instability (CIN)—in fast proliferating cancer cells is in a marked contrast with the fact that aneuploid cells do not efficiently proliferate. Normally, the proliferation of missegregating cells is limited by p53 activation (2). Accordingly, tumors with mutations in TP53 are often highly aneuploid, thus suggesting that the inactivation of p53 is essential for survival of aneuploids. However, aneuploidy is not always accompanied by p53 inactivation. In fact, in colorectal cancer (CRC), where aneuploidy is frequent, p53 inactivation occurs rather late during tumorigenesis (3). Thus, additional factors might guard the cells against aneuploidy and their mutations might provide tolerance to chromosome missegregation irrespective of p53 inactivation. Since aneuploidy is considered a promising target for cancer therapy, it is of importance to identify the factors that contribute to aneuploidy tolerance in cancer cells.
In past years, the advent of next generation sequencing (NGS) technologies has allowed extensive molecular characterization of several cancer types and identification of genetic alterations that underlie tumorigenesis as well as the intrinsic intra-tumor heterogeneity. In a paper recently published in Cancer Cell, López-García and colleagues took advantage of such technologies to identify mutations enriched particularly in aneuploid cells (4). In their study, the authors focused on CRC that can be classified into two major categories based on their genomic integrity. The so-called microsatellite instability (MSI) high tumors show genetic hypermutability manifested as single base mismatches or short insertions and deletions, while their chromosome content remains nearly diploid. In contrast, microsatellite stable (MSS) CRC tumors are highly chromosomally unstable and develop varying degrees of aneuploidy. In their study, a cohort of 17 MSS adenocarcinomas were grouped according to their degree of aneuploidy as aneuploid (10 samples) and diploid (7 samples) tumors by DNA image cytometry and centromeric fluorescence in situ hybridization. In addition to these tumor samples, MSS CRC cell lines, whose ploidy status was obtained from literature, were also used in the study. To identify aneuploidy-specific mutations, authors performed whole exome sequencing from these tumors and cell lines. The analysis confirmed several previously known CRC drivers such as mutations in APC, FBXW7 and KRAS. Additionally, their study revealed mutations in genes that were exclusively enriched in aneuploid tumors, among them, consistent with the earlier observations, TP53 mutations.
Next to the already known factors, the authors identified inactivating mutations of BCL9L to be enriched in aneuploid tumors. BCL9L is a transcriptional activator known for its role in regulating Wnt signaling. Together with its binding partners β-catenin and Pygo, BCL9L controls the transcriptional activity of TCF/LEF family of transcription factors. Markedly, the authors found that RNAi silencing of BCL9L promotes aneuploidy tolerance to naturally occurring chromosome segregation errors as well as to miss-segregation after treating cells with Mps1 inhibitor, reversine, which impairs the spindle assembly checkpoint activity. Interestingly, BCL9L silencing provided additive aneuploidy tolerance to the cells lacking p53. Moreover, shBCL9L cells that were pretreated with reversine to induce CIN engrafted better in immunocompromised mice and had increased karyotypic diversity compared to untreated and WT cells. This remarkable aneuploidy-suppressing function and the fact that BCL9L is the second most commonly truncated gene in aneuploid CRC prompted the authors to investigate BCL9L mutations in a large CRC tumor cohort dataset available from The Cancer Genome Atlas (TCGA). This analysis revealed that tumors harboring BCL9L mutations are highly chromosomally unstable. Strikingly, most of the tumors with BCL9L mutations retained one wild type allele of BCL9L, thus suggesting that a loss of one allele of BCL9L is sufficient to provide aneuploidy tolerance in tumors.
What molecular processes underlie the BCL9L mediated aneuploidy tolerance? It is well-known that chromosome missegregation induces p53 stabilization. In contrast, BCL9L silencing strongly reduced p53 accumulation following chromosome missegregation. But how can BCL9L activity affect p53 stabilization? The steady state levels of p53 in healthy cells are regulated by MDM2, an E3 ubiquitin ligase that tags p53 protein for proteasomal degradation. Recent studies have shown that an MDM2 fragment that arises from caspase-2 mediated cleavage can physically interact with p53, which results in p53 stabilization (5,6). Indeed, the authors observed caspase-2-mediated MDM2 cleavage in the cells treated with reversine, which was reduced upon BCL9L silencing, thus reducing p53 stabilization. Caspase-2 is a highly conserved member of caspases, a family of proteases involved in initiation and execution of apoptosis. Activated caspase-2 dimerizes and promotes its own cleavage generating a mature active form of caspase-2 consisting of p19/p32 heterodimer. Upon reversine treatment, caspase-2 became activated in p53 proficient cells and to a lesser extent in p53 deficient cells, while depletion of BCL9L reduced active caspase-2 in both TP53 WT and null cells.
Interestingly, BCL9L silencing facilitates aneuploidy tolerance also in p53 deficient cells. Here again caspase-2 seems to play a role. In this case, the caspase-2-mediated cleavage of cytosolic proapoptotic factor BH3 interacting domain death agonist (BID) to tBID, which translocates to mitochondria and induces mitochondrial apoptotic pathway, was reduced in misssegregating cells where BCL9L was silenced. The importance of caspase-2 mediated cell death in limiting survival after mitotic aberrations has been recently documented in other experimental settings (7,8). The observations of López-Garcí and colleagues strengthen the role of caspase-2 in suppressing endogenous as well as drug-induced chromosome segregation errors in cancer cells (Figure 1).
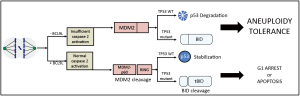
Finally, since BCL9L is involved in Wnt pathway regulation, authors hypothesized that BCL9L loss induces aneuploidy tolerance by negatively regulating Wnt signaling. Indeed, caspase-2 is also a target of β-catenin/TCF4 transcriptional complex and BCL9L silencing attenuates the TCF4 transcriptional activity, thereby reducing caspase-2 expression. Interestingly, cells treated with an inhibitor of Wnt signaling also reduced caspase-2 expression and became more tolerant to aneuploidy. Overactivation of Wnt signaling has been recognized as essential for the progression of CRC (9). Aberrantly increased Wnt signalling can be mediated by BCL9L overexpression, as it has been observed previously in CRC (10,11). The new findings suggest that downregulation of Wnt signaling via lack of BCL9L promotes aneuploidy tolerance and adds a new and unexpected layer of complexity to the role of Wnt signaling in tumorigenesis that should be investigated in future.
Although the p53 stabilization following chromosome missegregation has been well established, the underlying mechanisms and upstream signals remain unclear. Reactive oxygen species (12,13), DNA damage (14) and histone phosphorylation (15) were all proposed to activate p53 upon mitotic errors. Here, the authors suggest that BCL9L might act as a sensor of chromosome missegregation, which in turn prevents aneuploidy by stabilizing p53, or, in a p53 independent path, by increasing BID activity. The p53 independent pathway could act as a fail-safe mechanism against aneuploidy in the absence of p53. Additionally, caspase-2 may act upstream of p53 and be responsible for stabilizing p53 in cells that missegregated chromosomes. However compelling this idea seems at present, the routes that lead to caspase-2 activation remain to be elucidated. Once the mechanisms for caspase-2 activation in the context of chromosome missegregation are understood, drugs enhancing caspase-2 activity could potentially be used for treating chromosomally unstable tumors.
Aneuploidy is one of the hallmarks of cancer. Mounting evidence suggests that aneuploidy often comes hand in hand with ongoing CIN, thus driving intra-tumor heterogeneity and adversely affecting the prognosis of patients. TP53 has been established to protect cells from chromosome copy number changes, but this study points out that p53 is not alone in guarding cells against aneuploidy. Cancer cells need mechanisms such as BCL9L silencing or TP53 mutations in order to cope with ongoing CIN and to tolerate aneuploidy. Improved insight into these mechanisms will reveal novel tumor vulnerabilities and help to develop compounds that can target one of the most common and generic feature of cancer-aneuploidy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Zhenyu Lin (Cancer center, Union hospital, Huazhong University of Science and Technology, Wuhan, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.08.37). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swanton C, Nicke B, Schuett M, et al. Chromosomal instability determines taxane response. Proc Natl Acad Sci U S A 2009;106:8671-6. [Crossref] [PubMed]
- Thompson SL, Compton DA. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J Cell Biol 2010;188:369-81. [Crossref] [PubMed]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759-67. [Crossref] [PubMed]
- López-García C, Sansregret L, Domingo E, et al. BCL9L Dysfunction Impairs Caspase-2 Expression Permitting Aneuploidy Tolerance in Colorectal Cancer. Cancer Cell 2017;31:79-93. [Crossref] [PubMed]
- Oliver TG, Meylan E, Chang GP, et al. Caspase-2-mediated cleavage of Mdm2 creates a p53-induced positive feedback loop. Mol Cell 2011;43:57-71. [Crossref] [PubMed]
- Terry MR, Arya R, Mukhopadhyay A, et al. Caspase-2 impacts lung tumorigenesis and chemotherapy response in vivo. Cell Death Differ 2015;22:719-30. [Crossref] [PubMed]
- Dawar S, Lim Y, Puccini J, et al. Caspase-2-mediated cell death is required for deleting aneuploid cells. Oncogene 2017;36:2704-14. [Crossref] [PubMed]
- Fava LL, Schuler F, Sladky V, et al. The PIDDosome activates p53 in response to supernumerary centrosomes. Genes Dev 2017;31:34-45. [Crossref] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330-7. [Crossref] [PubMed]
- Adachi S, Jigami T, Yasui T, et al. Role of a BCL9-related beta-catenin-binding protein, B9L, in tumorigenesis induced by aberrant activation of Wnt signaling. Cancer Res 2004;64:8496-501. [Crossref] [PubMed]
- Sakamoto I, Ohwada S, Toya H, et al. Up-regulation of a BCL9-related beta-catenin-binding protein, B9L, in different stages of sporadic colorectal adenoma. Cancer Sci 2007;98:83-7. [Crossref] [PubMed]
- Li M, Fang X, Baker DJ, et al. The ATM-p53 pathway suppresses aneuploidy-induced tumorigenesis. Proc Natl Acad Sci U S A 2010;107:14188-93. [Crossref] [PubMed]
- Kuffer C, Kuznetsova AY, Storchová Z. Abnormal mitosis triggers p53-dependent cell cycle arrest in human tetraploid cells. Chromosoma 2013;122:305-18. [Crossref] [PubMed]
- Ohashi A, Ohori M, Iwai K, et al. Aneuploidy generates proteotoxic stress and DNA damage concurrently with p53-mediated post-mitotic apoptosis in SAC-impaired cells. Nat Commun 2015;6:7668. [Crossref] [PubMed]
- Hinchcliffe EH, Day CA, Karanjeet KB, et al. Chromosome missegregation during anaphase triggers p53 cell cycle arrest through histone H3.3 Ser31 phosphorylation. Nat Cell Biol 2016;18:668-75. [Crossref] [PubMed]


