
Evaluating treatment effects of gastric gastrointestinal stromal tumors between open and endoscopic ultrasonography guided endoscopy resection approaches: revealing minimally invasive, fast recovering and economical surgery for patients
Introduction
Gastrointestinal stromal tumor (GIST) is a rare kind of stromal neoplasm representing one of the most common mesenchymal tumor types of the gastrointestinal (GI) tract. It is firstly described by Mazur and Clark as early as 1983 (1). The estimated frequency of GIST is 10–20 cases per million populations (2,3). GIST accounts for less than 1% of all gastrointestinal tumors (GITs) and a majority of the lesions are benign with about 20–30% possibly malignant (3,4). GIST is hypothesized to originate from the interstitial cells of Cajal (ICC), the gut motility coordinator, which belongs to the autonomic nervous system of the intestine (5,6). Therefore, GIST could arise from anywhere along the GI tract from the esophagus to the rectum, usually from the muscularis mucosa or propria layers of the GI wall (7), but rarely found in the peritoneum, mesentery or omentum (8).
Clinical manifestations of GISTs are dependent on tumor size and location, therefore highly variable. GIST symptoms include melena, hematemesis, abdominal pain, discomfort, fullness, early satiety and palpable mass, although many GISTs exhibit no symptoms and are incidentally identified by imaging or endoscopy (6,9-14). Malignant GISTs often have local or distant metastasis when diagnosed, with the liver being the most frequent site and the bone, peritoneum, lung, pleura and subcutaneous tissue being common sites (15).
Most GISTs locate in the stomach (2) and is characterized by indolent clinical symptoms despite of possibilities for life-threatening emergency. GISTs bearing tumor size larger than 4 cm may display as abdominal emergencies, including GI hemorrhage (usually due to pressure necrosis and ulceration of overlying mucosa), intestinal obstruction or perforations.
The introduction of minimally invasive surgery has led to reduced post-operative stress and shorter hospital stay. In gastric surgery, the laparoscopic approach was correlated with rapid recovery, low morbidity, and decreased length of hospital stay compared with laparotomy (16,17). Similar to laparoscopy, endoscopy is considered as a minimally invasive approach and mostly used in diagnosis (18). However, endoscopy resections (ERs) are performed to remove small lesions (19). With guidance of endoscopic ultrasonography (EUS), the surgical process is hypothesized to be more improved and accurate (20). Hwang et al. evaluated sub epithelial GIST masses diagnosed by esophagogastroduodenoscopy (EGD), sigmoidoscopy or colonoscopy prospectively (21). It has also been reported that EUS retains 92% sensitivity and 100% specificity in differentiating submucosal tumor from extrinsic compression (22).
In our study, 77 patients with GIST were enrolled and divided randomly into two groups, the open surgery group (OS group) and the EUS guided ER group. The clinical outcomes were compared between the two groups.
Methods
Patients and study design
The study was performed in 77 GIST cases (OS + ER group, n=77) in the No. 1 People’s Hospital of Yunnan, China between Jan 2012 to Dec 2016, including 39 male patients and 38 female patients within the age range of 58.2±7.6 years. All procedure and data collection are aligned and complied with national guidelines. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. All patients included developed single tumor without metastasis. GISTs were diagnosed and confirmed by ultrasonography, computed tomography (CT), endoscopy and EUS. Thirty-six patients (21 males and 15 females) were treated with conventional laparotomy or OS group and 41 patients (18 males and 23 females) were treated with EUS assisted endoscopy (ER group). Patients were followed up from 0.5 to 4 years post operation with detailed information recorded: the post-operative recovery and complications were traced and summarized for analysis. The protocol for general anesthesia and post-operative pain relief was identical in all patients.
Tumors evaluated by EUS had to satisfy the following criteria for entry into study:
- Tumors with irregular margins (heterogeneous, lobulated, regional lymphadenopathy, ulceration, extraluminal) and high echo area (cystic region and liquefied) during EUS scan were excluded;
- Tumors sizing from 1.5-4 cm as a bulge with a smooth, intact, normal appearing mucosa in the GI tract were included;
- Tumors were located in gastric muscularis mucosa, submucosa and propria. EUS assisted ER surgical methods include:
- endoscopic submucosal dissection (ESD);
- endoscopic full-thickness resection (EFR);
- submucosal tunneling endoscopic resection (STER).
Statistical analysis
The Chi-square statistics were used in categorizing variables comparison with frequencies presented by percentages. Continuous variables were expressed as mean ± standard deviation (SD) or median. The comparisons were performed by the independent samples t-test or the Mann-Whitney U-test. P value <0.05 was regarded as statistically significant.
Results
Seventy-seven patients were enrolled with entry into this study. The average age is 58.2±7.6 years overall. They were analyzed and divided into two groups: (I) treated with OS (OS group n=36, male n=21, female n=15); (II) treated with EUS-guided ER (ER group n=41, male n=18, female n=23). The relevant characteristics of patients and the types of surgical approaches are summarized in Tables 1 and S1. The average age is 61.1±6.7 in the OS group and 52.2±4.1 in the ER group. The statistical analysis of age, gender, tumor size (tumor length between 2–4 cm) and tumor location suggested similar patient characteristics between the two groups with all P>0.05. According to National Institutes of Health (NIH) criteria for classification, the GISTs were classified into very low, low, intermediate and high risks post resection (23,24). In OS group, there were 4 very low risk cases, 20 low risk cases, 11 intermediate cases and 1 high risk case. In ER group, there were 8 very low risk cases, 30 low risk cases and 3 intermediate cases. Regardless of the 1 high-risk case in the OS group, both groups contain similar number of different grades of GISTs (P>0.05), therefore comparable in other parameters.
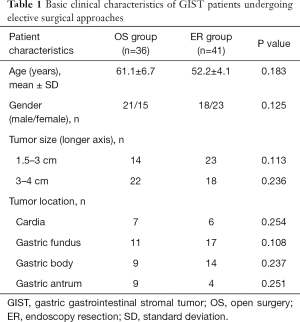
Full table
The OS group showed significantly longer operative time in surgical procedure (95.1±11.6 min) in contrast to the ER group (82.8±10.2 min). The estimated blood loss during surgery was significantly less in the ER group (39.8±7.2 mL) compared to the OS group (112.8±17.6 mL). All patients from both groups had complete resection with negative margins. The post-operative fasting and post-operative hospitalization are both shorter in the ER group significantly (Table 2), indicating a quicker recovery and better clinical outcome for the ER group.

Full table
Given that OS costs more than normal EUS-guided ER, we compared the cost and expenses of treatment mainly by examining the total hospitalization days and post-operative hospitalization days. Also, we calculated total expenses and expenses for medicine only. The ER group exhibited significantly fewer days not only in total hospitalization days, but also in post-operative hospitalization days. Consistently, the expenses only for medicine and the total expenses were significantly lower in the ER group, partially due to shortened hospitalization and reduced steps of procedures (Table 3), suggesting that EUS-guided ER is an economic surgical option in GIST patients.

Full table
Case presentation
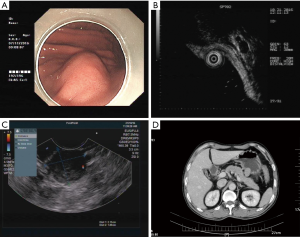
In all of the successful ER treated patients, we would like to report one of the cases. A 48-year-old Chinese man was first admitted due to upper right abdominal pain under pressure. EUS detected a bulging mass in the greater curvature of the stomach (Figure 1A), located in the muscularis propria (Figure 1B,C). Abdominal CT confirmed a solid mass measuring approximately 2.5 cm × 2.0 cm (Figure 1D). The tumor was diagnosed as GIST growing outward from the gastric wall.
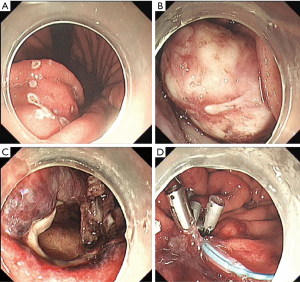
EFR was performed under the guidance of EUS. Operative findings revealed that the tumor as a hard mass firmly attached to the greater curvature of the stomach closed to the posterior gastric wall (Figure 2A,B). The branches of the right and left gastroepiploic arteries fed the tumor, which required clipping of arteries during the process. After the tumor was detached together with the full-thickness (Figure 2C), the incision was sutured leaving a titanium clips to fix the suture (Figure 2D).
The tumor measured 28 mm × 20 mm × 18 mm, and the cut surface of the resected specimen was pink, smooth and uniform (Figure 3A). The tumor was then sectioned for pathological analysis. Under bright field for hematoxylin and eosin (H & E) staining, the tumor sample revealed proliferating of spindle-shaped cells, a typical tissue feature for GISTs (Figure 3B). Immuno-histological examination showed that the tumor was negative for PCK, desmin, S100 and positive for Vimentin, CD34, CD117 and Anoctamin 1, consistent with the diagnosis and grading into intermediate GIST (Figure 3C and data not shown).
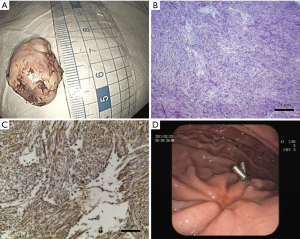
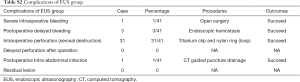
Patients were hospitalized from 5–8 days for additional observation for complications such as delayed bleeding, perforation and infections, since resection of small tumors would likely lead to high incidence of gastric wall perforation (Table S2). The patient’s post-operative course was uneventful. There were no clinical complications during post-operative hospitalization and no sign of tumor recurrence was observed for 5 months after operation. The examination follow-up 3 months after operation exhibited good recovery at the suture with the titanium clip attached (Figure 3D).
Discussion
GISTs are rare tumors with high index of suspicion for diagnosis (3,6,25). A combined diagnosis procedure is normally used since none of the single diagnostic procedure such as CT, ultrasonography and magnetic resonance imaging pertains a 100% certainty (2,3). Although, preoperative fine needle aspiration has the risk of tumor rupture, recent reports have shown up to 89% accuracy by EUS guided fine needle aspiration, indicating the refined diagnostic results by aid of EUS (26).
The most common arising site (60–70%) for GIST is the stomach (2). Thus our study mainly focused on the GISTs happening in gastric area. The standard treatment of choice is complete surgical resection as soon as presentation/identification of the tumor (27), with 5-year survival of 48–65%, partially due to poor response to chemotherapy or radiotherapy (2,3,28,29). Therefore, surgical approaches should be carefully reviewed in each case before the removal of the GIST patients to favor a better clinical outcome and lighter economic pressure to the families.
In this study, we enrolled 77 patients to compare two major surgical approaches for GIST tumors within length of 1.5–4 cm. In comparison to conventional OS, patients treated with EUS-guided ER showed significantly less time in surgery procedure, less operative blood loss, less complication rates, less duration for postoperative fasting and postoperative hospitalization, indicating a quicker recovery and better clinical outcome. Furthermore, treatment group with ER displayed reduced expense of hospitalization due to lower procedure expense and shorter hospital stay with rare tumor recurrence. Our one successful case indicated EUS-guided ER as a favored, promising, minimally invasive and economic approach for non-high risk GIST patients, paving the road for future patient/doctor references in utilizing EUS-guided ER treatment.
In summary, EUS-guided endoscopic tumor resection is an effective method to remove 1.5–4 cm non-high risk GISTs in patients comparing to OS, and may be beneficial to patients economically.

Full table
Full table
Acknowledgments
Funding: This research was supported by grants from the National Natural Science Foundation of China (NSFC; grant No. 81260077 to H Fan and grant No. 81560107 to Z Song).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.08.35). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol 1983;7:507-19. [Crossref] [PubMed]
- Connolly EM, Gaffney E, Reynolds JV. Gastrointestinal stromal tumours. Br J Surg 2003;90:1178-86. [Crossref] [PubMed]
- Efremidou EI, Liratzopoulos N, Papageorgiou MS, et al. Perforated GIST of the small intestine as a rare cause of acute abdomen: Surgical treatment and adjuvant therapy. Case report. J Gastrointestin Liver Dis 2006;15:297-9. [PubMed]
- Sandvik OM, Soreide K, Kvaloy JT, et al. Epidemiology of gastrointestinal stromal tumours: single-institution experience and clinical presentation over three decades. Cancer Epidemiol 2011;35:515-20. [Crossref] [PubMed]
- Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998;152:1259-69. [PubMed]
- Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors presenting as omental masses--a clinicopathologic analysis of 95 cases. Am J Surg Pathol 2009;33:1267-75. [Crossref] [PubMed]
- Lin YM, Chiu NC, Li AF, et al. Unusual gastric tumors and tumor-like lesions: Radiological with pathological correlation and literature review. World J Gastroenterol 2017;23:2493-504. [Crossref] [PubMed]
- Reith JD, Goldblum JR, Lyles RH, et al. Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome. Mod Pathol 2000;13:577-85. [Crossref] [PubMed]
- Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc 1999;74:543-52. [Crossref] [PubMed]
- DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8. [Crossref] [PubMed]
- Levy AD, Patel N, Abbott RM, et al. Gastrointestinal stromal tumors in patients with neurofibromatosis: imaging features with clinicopathologic correlation. AJR Am J Roentgenol 2004;183:1629-36. [Crossref] [PubMed]
- Jiang ZX, Zhang SJ, Peng WJ, et al. Rectal gastrointestinal stromal tumors: imaging features with clinical and pathological correlation. World J Gastroenterol 2013;19:3108-16. [Crossref] [PubMed]
- Salvi PF, Lorenzon L, Caterino S, et al. Gastrointestinal stromal tumors associated with neurofibromatosis 1: a single centre experience and systematic review of the literature including 252 cases. Int J Surg Oncol 2013;2013:398570.
- Sorour MA, Kassem MI, Ghazal Ael H, et al. Gastrointestinal stromal tumors (GIST) related emergencies. Int J Surg 2014;12:269-80. [Crossref] [PubMed]
- Burkill GJ, Badran M, Al-Muderis O, et al. Malignant gastrointestinal stromal tumor: distribution, imaging features, and pattern of metastatic spread. Radiology 2003;226:527-32. [Crossref] [PubMed]
- Kim YW, Baik YH, Yun YH, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg 2008;248:721-7. [Crossref] [PubMed]
- Park JM, Jin SH, Lee SR, et al. Complications with laparoscopically assisted gastrectomy: multivariate analysis of 300 consecutive cases. Surg Endosc 2008;22:2133-9. [Crossref] [PubMed]
- Gao Z, Wang C, Xue Q, et al. The cut-off value of tumor size and appropriate timing of follow-up for management of minimal EUS-suspected gastric gastrointestinal stromal tumors. BMC Gastroenterol 2017;17:8. [Crossref] [PubMed]
- Jenssen C, Barreiros AP, Will U, et al. German Survey on EUS-Guided Diagnosis and Management of Gastrointestinal Stromal Tumors (GISTs) - Evidence or "Gut-Feeling"? Ultraschall Med 2015;36:494-500. [Crossref] [PubMed]
- Lakhtakia S, Seo DW. Endoscopic ultrasonography-guided tumor ablation. Dig Endosc 2017;29:486-94. [Crossref] [PubMed]
- Hwang JH, Saunders MD, Rulyak SJ, et al. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc 2005;62:202-8. [Crossref] [PubMed]
- Rosch T, Kapfer B, Will U, et al. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol 2002;37:856-62. [Crossref] [PubMed]
- Lanke G, Lee JH. How best to manage gastrointestinal stromal tumor. World J Clin Oncol 2017;8:135-44. [Crossref] [PubMed]
- von Mehren M, Randall RL, Benjamin RS, et al. Soft Tissue Sarcoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:758-86. [Crossref] [PubMed]
- Sornmayura P. Gastrointestinal stromal tumors (GISTs): a pathology view point. J Med Assoc Thai 2009;92:124-35. [PubMed]
- Vander Noot MR 3rd, Eloubeidi MA, Chen VK, et al. Diagnosis of gastrointestinal tract lesions by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer 2004;102:157-63. [Crossref] [PubMed]
- Chen TW, Liu HD, Shyu RY, et al. Giant malignant gastrointestinal stromal tumors: recurrence and effects of treatment with STI-571. World J Gastroenterol 2005;11:260-3. [Crossref] [PubMed]
- Judson I. Gastrointestinal stromal tumours (GIST): biology and treatment. Ann Oncol 2002;13:287-9. [Crossref] [PubMed]
- Aparicio T, Boige V, Sabourin JC, et al. Prognostic factors after surgery of primary resectable gastrointestinal stromal tumours. Eur J Surg Oncol 2004;30:1098-103. [Crossref] [PubMed]


