
“Wonder drugs” in central nervous system lymphoma
Approximately one-quarter of patients with primary central nervous system lymphoma (PCNSL) do not respond to first-line therapy and more than one-half relapse (1). No optimal therapy has been established for these patients. Re-exposure to high-dose methotrexate (HDMTX) is an effective option for patients who experienced a long lasting remission after primary therapy with this drug (2). In studies using other classical cytostatics with or without rituximab, a wide range of responses of 14% to 53% has been reported with, however, usually poor long-term control reflected by a relatively short median progression-free survival (PFS) of 2 to 5 months only (3-7). Whole-brain radiotherapy (WBRT) is probably the most active salvage treatment in PCNSL with response rates of 60% to 79% and median PFS of approx. 10 months after retreatment (8,9), however, with a major risk of CNS toxicity, particularly in elderly patients. In younger and fit patients, high-dose chemotherapy followed by autologous stem-cell transplantation is frequently considered, however, the results of the recent prospective study were rather moderate with a response rate of 56%, a toxicity-related death rate of 10% and a median PFS of 12.4 months in a relatively young (median age 65 years, maximum 75 years) and otherwise healthy patients’ population (10).
Considering the poor prognosis of relapsed/refractory (r/r) PCNSL with therapy options currently available, there is an understandable desire for new, more effective and less toxic treatments. The immunosuppressive microenvironment is currently one of the targets most frequently addressed in PCNSL.
Until recently, CNS had been assumed as “immune privileged” organ lacking immunosurveillance. This dogma was challenged 2015 when a CNS lymphatic system was discovered. Within this system, CNS antigens and T cells can reach the deep cervical lymph nodes through cerebrospinal fluid (CSF)-filled channels (11), migrated antigen presenting cells (APC) from the CNS can present antigens to T-cells and return to the CNS perivascular spaces. Injury, inflammation and tumor can cause disruption and increased permeability of blood-brain barrier (BBB) and contribute to the interaction between the CNS and immune system. Moreover, it has been demonstrated that in various neurological diseases CNS is permissive for antigen-specific immunity from periphery, and that a lymphatic system exists in which leukocytes can be shuttled by lymphatic vessels to CNS with an intact BBB (12). Taken together, CNS can actively communicate with the immune system, which theoretically can be used for therapeutic purposes.
Immunotherapy with checkpoint inhibitors showed remarkable success in recent years in many tumors. Immune checkpoint inhibitors bind with high affinity to programmed death ligand (PD) receptors on T-cells, blocking their interaction with programmed death ligand ligands 1 and 2 (PD-L1/PD-L2) and restoring T-cell antitumor function. Benefits with immune checkpoint inhibitors for patients with brain tumors, both primary such as glioblastoma and brain metastases have been demonstrated in several small studies. This led to initiation of numerous phase I-III trials with different PD-1-inhibitors alone or combined with vascular endothelial growth factor (VEGF)-blockade, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)-blockade, classical chemotherapy or radiotherapy, both in the up-front and recurrent setting.
In PCNSL, an immunohistologic study on 20 cases revealed expression of PD-L1 or PD-1 on tumor cells, tumor infiltrating lymphocytes (TIL) or tumor associated macrophages (TAM) in 90% of cases; 60% showed positivity of TIL for PD-1 and 20% positivity of TAM for PD-L1 (13). Molecular analyses confirmed the frequent 9p24.1 (the PD-L1/PD-L2 locus) alterations and, less frequently, chromosomal rearrangements involving PD-L1 or PD-L2, both resulting in increased expression of PD-L1 and PD-L2 in these tumors (14). All this raised hope and enthusiasm that immune check point inhibitors may prove effective in PCNSL.
In the first published study with check-point inhibitors in CNS lymphoma, Nayak et al. report on a small series of 5 patients, 4 with r/r PCNSL and one with a CNS relapse of a primary testicular lymphoma (PTL) treated with the check-point inhibitor nivolumab. Although it is not clear how patients were selected for nivolumab treatment and what in detail their pretreatment was, the collective seems representative considering age and performance status (median age 64 years, range 54–85, median KPS 70%). An impressive response rate of 100% was reported with 4 complete responses (CR) and one partial response (PR) and three patients remaining progression-free at 13+ to 17+ months. The response evaluation, however, was not straight forward for several reasons. First, two patients received radiation immediately prior to the initiation of nivolumab, so it cannot be excluded that the response attributed to the drug represented the delayed-effect of radiotherapy. Secondly, it is not clear how many patients were pretreated with steroids shortly before nivolumab (it is only said that one patient was on dexamethasone at the time of starting nivolumab). This is important since steroids may have a very profound and sometimes long-lasting effect in CNSL. Thirdly, in one patient, no contrast-enhanced MRI was possible, making the interpretation of response rather difficult. Toxicity seems to have been tolerable (grade 2 pruritus in one patient and grade 2 fatigue in another patient), however, it is not clear if a detailed monitoring and documentation of adverse effects was performed. Moreover, one patient developed grade 4 renal insufficiency which did not improve despite drug discontinuation and treatment with steroids. Since immunotherapies can cause interstitial nephritis in at least 2% of patients (15), nivolumab toxicity cannot be excluded in this patient even if renal biopsy was not conclusive. It would have been interesting to look for a correlation between PD-L1 or PD-1 expression and response quality, but no data on tumor tissue was given. Altogether, these very preliminary results are promising but should be interpreted with caution, and a confirmation by a well-designed controlled prospective study with a clear definition of inclusion and exclusion and response criteria as well as detailed monitoring for toxicity is mandatory before nivolumab is given outside a clinical trial.
The (immunosuppressive) tumor microenvironment is also a target for lenalidomide—another “targeted drug” evaluated in clinical trials in PCNSL. Response to lenalidomide was seen in three of 6 mostly elderly and intensively pretreated PCNSL patients. However, two of the responses were very short and possibly attributed to the simultaneous steroids application (16). In a phase I study (17), of 13 patients with refractory PCNSL treated with different lenalidomide doses in combination with rituximab, 9 (69%) responded with 6 responses having been durable beyond 6 months; the median PFS was, however, very short with 1.5 months only. Interestingly, a dose-dependent penetration of lenalidomide into ventricular CSF was found, with the CSF plasma partition coefficient achieving 20% or more at the 15- and 20-mg doses. Two dose-limiting toxicities occurred at the 20-mg dose level, consisting of a grade 4 infection and grade 3 confusion.
A trial published thus far in abstract form only used lenalidomide combined with rituximab followed in responders by lenalidomide maintenance. Of the 50 patients included, 34 (68%) received concomitant corticosteroids during the first month of treatment, which, as mentioned above, impedes interpretation of response data. At the end of the induction phase, response was found in 17 patients (39%) including 13 CR (30%). Only 17 patients started the maintenance phase. Grade 3 or 4 adverse events, mostly infections, were reported in 11 patients. Moreover, a second cancer (melanoma) occurred in one patient. With a median follow-up of 9 months median PFS of the whole population was 8.1 months (18).
As the insight into the molecular pathogenesis of PCNSL grows, there is an increasing number of possible targets for specific therapy. Frequent mutations of the B-cell receptor (BCR) subunit CD79B and the Toll-like receptor (TLR) adaptor protein MYD88 suggest that PCNSL may be addicted to BCR signaling. Thus, the phosphatidylinositol- 4,5-bisphosphate 3-kinase/mammalian target of rapamycin 1/2 (PI3K/mTOR) and TLR/BCR/NF kappaB pathways are the other therapeutic targets most extensively being evaluated in clinical studies in PCNSL.
The allosteric mTOR inhibitor temsirolimus was the first target drug prospectively evaluated in r/r PCNSL (1). In a relatively old (median age 70 years, maximum 83 years) and heavily pretreated patients’ cohort, a relatively high-response rate of 54% was achieved. Toxicity, however, was considerable with grade 3–4 hyperglycemia in 30% of patients, thrombocytopenia in 22% and infection in 19%. Measurable temsirolimus/sirolimus concentration was found in the CSF of one patient only, rising up the question how the tumor was reached by the drug. Disappointingly, responses were frequently short and the median PFS was 2.1 months only. Inhibition of the activity of mTOR within the TORC1 complex only with activation of TORC2 was proposed as a putative resistance mechanism to temsirolimus. A simultaneous PI3K inhibition could help reduce subsequent AKT activation which can bypass some effects of mTOR inhibition. A study with a dual PI3K-mTOR inhibitor in r/r PCNSL is currently ongoing (see Table 1).
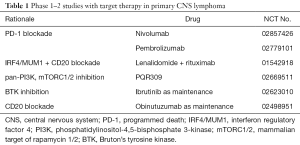
Full table
Ibrutinib, an inhibitor of the bruton tyrosine kinase (BTK) positioned early within the BCR signaling cascade and linking the BCR and the NF-kappaB pathway, is currently the most extensively evaluated drug in PCNSL. Ibrutinib produced rapid response in all three patients with CNS relapse of mantle-cell lymphoma without severe toxicity. Remarkably, CSF penetration of ibrutinib was demonstrated with a CSF-to-plasma concentration ratio of 1–7% (19). Consequently, single agent ibrutinib 840 mg was evaluated in a phase I-trial with 20 patients with r/r PCNSL and secondary CNS lymphoma (SCNSL). Response was found in 10/13 (77%) PCNSL patients and 5/7 (71%) SCNSL patients; the median PFS was 4.6 and 7.4 months, respectively (20). Toxicity was stated to be manageable although grade 3–4 infection occurred in 7 (35%) of patients. Of 15 responders, one was on dexamethasone at enrollment and three discontinued dexamethasone 4 weeks bevor enrollment; since it was not said, what the interval between enrollment and first response evaluation was, the effect of steroids on response cannot be excluded in these patients. This study provides a first glimpse into genetic mechanisms of “de novo” ibrutinib resistance pointing to CARD11 mutations which promote BTK-independent NF-kappaB activation. Moreover, CD79B mutations which were frequently associated with MYD88 mutations appeared to attenuate BTK addiction by providing a redundant survival signal. Interestingly, it was suggested that survival signal provided by the PI3K/mTOR axis was at least partially independent from the BTK/NF-kappaB signaling which suggests that it may be reasonable to combine ibrutinib with PI3K/mTOR inhibitors in PCNSL.
One study evaluating single agent ibrutinib 560 mg was published in abstract form only. In this trial, patients with recurrent PCNSL or ocular lymphoma were enrolled. In the first 18 patients three cases of CR and 7 of PR were registered, however, 5 patients received concomitant corticosteroids during the first month of treatment. At the time of analysis with a median follow-up of 6.6 months, 9 patients discontinued ibrutinib after a median duration of 3 months because of a disease progression in 8 and a concurrent illness in one. One patient experienced a pulmonary aspergillosis (21).
A study combining ibrutinib with classic chemo-/immunotherapy (temozolomide, etoposide, doxil, rituximab), dexamethasone and intraventricular cytarabine (DA-TEDDI-R) in both r/r PCNSL and newly diagnosed patients impressively illustrates the risk of a too ambitious handling of new drugs in PCNSL patients. HDMTX was not included in the treatment protocol due to less synergy with ibrutinib in pre-clinical experiments. Toxicity was dramatic and atypical: of 18 patients (5 of whom were untreated) who started treatment two developed fatal (grade 5) pulmonary/CNS aspergillosis already during the first treatment phase consisting of ibrutinib alone. Of the 16 patients who continued with chemo-/immunotherapy, 9 (56%) developed grade 3–4 pulmonary infection (including 5 cases of aspergillosis) and three (19%) other grade 3–4 infection (two combined CNS/pulmonary aspergillosis and one enterocolitis). Moreover, there were fatal febrile neutropenia, stroke and ventricular arrhythmia in one case each. Overall, rate of death on therapy was 45% (!) with 5 deaths due to toxicity and three due to tumor progression (22). This dramatic toxicity obviously overshadowed the response rate of 94% [unconfirmed (u)CR in 12 and PR in one patient of 14 evaluable patients].
Conducting prospective studies with PCNSL patients is challenging considering the rarity of this disease, its frequently aggressive course and patients’ fragility. Much as therapeutic progress is awaited, preliminary results obtained with novel drugs on limited numbers of selected patients should be interpreted with caution taking patients’ characteristics, concomitant therapy, methods of response evaluation and toxicity into consideration. A confirmation in adequately-sized and well-designed trials should be warranted before the wider use of new drugs can be recommended. Evaluation of CNS penetration and exploring molecular determinants of treatment response are highly desirable and should be important goals of these trials. Treating physicians should be cautious in turning in desperation to new “wonder drugs” despite lack of evidence of efficacy and tolerability.
A couple of studies evaluating novel drugs in r/r PCNSL is currently ongoing and their results are awaited tensely (Table 1).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Pei-Pei Xu (Department of Hematology, The Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, China).
Conflicts of Interest: The author received research support Pfizer, Mundipharma and Riemser.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Korfel A, Schlegel U, Herrlinger U, et al. Phase II Trial of Temsirolimus for Relapsed/Refractory Primary CNS Lymphoma. J Clin Oncol 2016;34:1757-63. [Crossref] [PubMed]
- Plotkin SR, Betensky RA, Hochberg FH, et al. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res 2004;10:5643-6. [Crossref] [PubMed]
- Fischer L, Thiel E, Klasen HA, et al. Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol 2006;17:1141-5. [Crossref] [PubMed]
- Enting RH, Demopoulos A, DeAngelis LM, et al. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology 2004;63:901-3. [Crossref] [PubMed]
- Arellano-Rodrigo E, López-Guillermo A, Bessell EM, et al. Salvage treatment with etoposide (VP-16), ifosfamide and cytarabine (Ara-C) for patients with recurrent primary central nervous system lymphoma. Eur J Haematol 2003;70:219-24. [Crossref] [PubMed]
- Maza S, Kiewe P, Munz DL, et al. First report on a prospective trial with yttrium-90-labeled ibritumomab tiuxetan (Zevalin) in primary CNS lymphoma. Neuro Oncol 2009;11:423-9. [Crossref] [PubMed]
- Mappa S, Marturano E, Licata G, et al. Salvage chemoimmunotherapy with rituximab, ifosfamide and etoposide (R-IE regimen) in patients with primary CNS lymphoma relapsed or refractory to high-dose methotrexate-based chemotherapy. Hematol Oncol 2013;31:143-50. [Crossref] [PubMed]
- Nguyen PL, Chakravarti A, Finkelstein DM, et al. Results of whole-brain radiation as salvage of methotrexate failure for immunocompetent patients with primary CNS lymphoma. J Clin Oncol 2005;23:1507-13. [Crossref] [PubMed]
- Hottinger AF, DeAngelis LM, Yahalom J, et al. Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma. Neurology 2007;69:1178-82. [Crossref] [PubMed]
- Kasenda B, Ihorst G, Schroers R, et al. High-dose chemotherapy with autologous haematopoietic stem cell support for relapsed or refractory primary CNS lymphoma: a prospective multicentre trial by the German Cooperative PCNSL study group. Leukemia 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015;523:337-41. [Crossref] [PubMed]
- Huang J, Liu F, Liu Z, et al. Immune Checkpoint in Glioblastoma: Promising and Challenging. Front Pharmacol 2017;8:242. [Crossref] [PubMed]
- Berghoff AS, Ricken G, Widhalm G, et al. PD1 (CD279) and PD-L1 (CD274, B7H1) expression in primary central nervous system lymphomas (PCNSL). Clin Neuropathol 2014;33:42-9. [Crossref] [PubMed]
- Chapuy B, Roemer MG, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood 2016;127:869-81. [Crossref] [PubMed]
- Cortazar FB, Marrone KA, Troxell ML, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016;90:638-47. [Crossref] [PubMed]
- Houillier C, Choquet S, Touitou V, et al. Lenalidomide monotherapy as salvage treatment for recurrent primary CNS lymphoma. Neurology 2015;84:325-6. [Crossref] [PubMed]
- Rubenstein JL, Fraser E, Formaker P, et al. Phase I investigation of lenalidomide plus rituximab and outcomes of lenalidomide maintenance in recurrent CNS lymphoma. J Clin Oncol 2016;34:abstr 7502.
- Ghesquieres H, Houillier C, Chinot O, et al. Rituximab-Lenalidomide (REVRI) in Relapse or Refractory Primary Central Nervous System (PCNSL) or Vitreo Retinal Lymphoma (PVRL): Results of a "Proof of Concept" Phase II Study of the French LOC Network. Blood 2016;128:785.
- Bernard S, Goldwirt L, Amorim S, et al. Activity of ibrutinib in mantle cell lymphoma patients with central nervous system relapse. Blood 2015;126:1695-8. [Crossref] [PubMed]
- Grommes C, Pastore A, Palaskas N, et al. Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma. Cancer Discov 2017;7:1018-29. [Crossref] [PubMed]
- Choquet S, Houillier C, Bijou F, et al. Ibrutinib Monotherapy in Relapse or Refractory Primary CNS Lymphoma (PCNSL) and Primary Vitreo-Retinal Lymphoma (PVRL). Result of the Interim Analysis of the iLOC Phase II Study from the Lysa and the French LOC Network. Blood 2016;128:784.
- Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell 2017;31:833-843.e5. [Crossref] [PubMed]


