
Notch signalling: the true driver of small cell lung cancer?
Introduction
Small cell lung cancer (SCLC), accounting for up to 15% of all cancers, is characterised by high grade neuroendocrine tumours with short doubling time, a high growth fraction and rapid metastatic spread (1). The dramatic lethal outcomes result from the inevitable development of a resistance to front line chemotherapy, possibly due to the expansion of the cancer cell subpopulation with stem cell like (2) and neuroendocrine properties (3). In the study by Dr. Julien Sage et al. entitled “Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer”, the authors demonstrate a direct action of the Notch pathway in the promotion of this resistant subpopulation (4). The Notch pathway is an evolutionarily conserved signalling system whose role in malignancy was first attributed to the presence of truncated protein, later named NOTCH-1, as a result of a t(7;9)(q34;q34.3) chromosomal translocation in over 65% of human T lymphoblastic leukemia (T-ALL) (5). The deregulation of the pathway has been associated with both oncogenic and tumour suppressive properties (6), and implicated in the regulation of angiogenesis and the release from tumour dormancy (7).
Dr. Sage et al. identify the transcriptional repressor Rest (also known as Nrsf) as a member of a molecular switch that blocks neuroendocrine gene expression in Notch-active small-cell lung cancer cells. Activation of this switch was associated with a reduction in cell growth and the emergence of chemoresistance, indicative of both a tumour suppressive and a pro-tumorigenic role of Notch in these cells. This work highlights a unique opportunity to develop novel biomarkers indicative of those patients with SCLC likely to benefit from Notch-inhibition combination strategies.
The Notch signalling pathway: activation and purpose
The Notch pathway is composed of ligands (Jagged 1 & 2, and Delta-like homologues 1–4) and receptors (Notch-1 to 4). Consisting of both extra- and intra-cellular domains, each receptor is cleaved by the intramembrane protease gamma-secretase following ligand binding. This enables the release of the Notch intracellular domain (NICD) and its nuclear translocation. Interaction with the transcriptional repressor RBPJ (recombining binding protein suppressor of hairless) triggers the induction of downstream target genes, such as HES and HEY (Figure 1) (8).
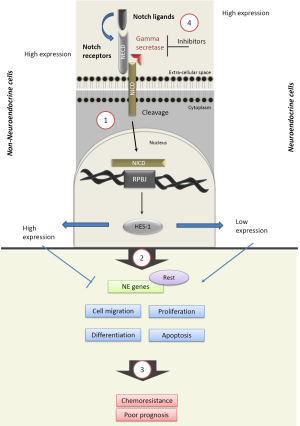
Structural differences between the four Notch receptors exist, with the Notch-3 receptor emerging as most distinct (9). For instance, the presence of both the transactivation domain (TAD) and cytokine response regions (NCR) post cleavage of the Notch-3 receptor (10), may explain the unique ability of Notch-3 to repress HES-1 signalling (11). But mutations in Notch receptor genes are also common in tumours, ranging from 7.3% of the 905 tumour tested displaying a mutation in Notch-1, down to 2.9% for Delta-like ligand 4 (12). The clinical significance of theses structural differences and mutational patterns remains poorly understood, but supports the presence of an heterogeneous cancer cell population, and may explain the observed variations in the expression of the pathway in cancer cells (13).
While the expression of Notch ligand, receptors and targets was detected in SCLC patient specimens by Dr. Sage (4) and others (14), in this cohort of 110 SCLC patients, 25% of tumours displayed an inactivating mutation in one of the NOTCH genes. In a murine model, the artificial activation of the Notch-1 and Notch-2 receptor, through the use of conditional expression of their NICD significantly reduced tumour growth (14). Loss of Notch activity is thus emerging as potentially key to the development of SCLC.
Biological characteristics of SCLC possibly regulated by Notch
The Notch pathway is likely involved in the regulation of the clinical behaviour of SCLC, through its action on a number of biological processes such as neuroendocrine differentiation (15), proliferation, cell adhesion and epithelial to mesenchymal transition (EMT) (16). This regulation may be largely mediated through the Notch-1 receptor, as demonstrated by a series of gene transfection and knockdown experiments (14,17,18). In mouse pulmonary cells, the loss of Notch expression was associated with an increase in the neuroendocrine markers achaete-scute complex homologue 1 (ASCL1) and insulinoma-associated protein 1 (INSM1) (15,19). The Notch-1 mediated regulation of EMT is proposed to promote cancer stem cells renewal (20) and could represent a mechanisms to the induction of chemoresistance (21) .
The role of ASCL1 in the development of SCLC is increasingly reported. Genetic profiling for ASCL-1 expression distinguishes “classic” from “variant”, neurogenic differentiation factor 1 (NEUROD1) expressing subtypes (22). ASCL-1 regulates the expression of a series of pro-oncogenes linked with SCLC progression and survival, such as BCL2 and SOX2 (23,24). Transfection of adenocarcinoma cell lines with ASCL1 leads to the induction of neuroendocrine phenotypes and loss of epithelial cell features (15). In Notch-expressing cells, an elevation in the expression levels of the inhibitory Notch ligand Delta-like protein 3 (DLL3) in most SCLCs has been linked to expression of ASCL-1 (24). As a result, the clinical potential of DLL3 inhibition was proposed and the antibody-drug conjugate rovalpituzumab tesirine was developed. Following encouraging results in vivo (25), the results of the first phase I clinical trial demonstrated the safety of this approach and report confirmed objective response in 18% of the 60 assessable patients (26).
Sage et al. elegantly describe a mechanism for the regulation of SCLC neuroendocrine and non-neuroendocrine subpopulations by Notch signalling. The authors used the triple negative p53flox/flox;Rbflox/flox;p130flox/flox TKO mouse model and first demonstrated, using a green fluorescent reporter gene under the control of the HES gene promoter (4), the presence of both GFP-positive and GFP-negative cells subpopulations. GFP-positive cells expressed higher levels of HES-1, the notch target Nrarp (Notch-regulated ankyrin-repeat protein), and the Notch1/2/3 receptors and reduced neuroendocrine genes expression compared to GPF negative, hence Notch inactive cells, who tended to overexpress most Notch ligands, including DLL3. These two subpopulations, displaying non-neuroendocrine (GFP-positive) and neuroendocrine (GFP-negative) features appeared to interact through the provision of Notch ligand by GFP-negative cells to the Notch receptor overexpressing GFP-positive cells, and as a result enabled a transition from a neuroendocrine to a non-neuroendocrine state in a process involving the transcription factor Rest. In this model, ASCL1 knockdown by itself failed to affect the expression of NE genes and cellular morphology and activate the transition switch (4).
The Notch-3 receptor may however also play an important role in SCLC tumorigenesis. Elevated in non-small cell lung cancer (NSCLC), as indicated by a meta-analysis involving 3,663 NSCLC patients (27), NOTCH-3 expression in the cancer tissue was weaker than that of the corresponding non-tumor tissue in SCLC patients (28). Evaluation of the consequences of Notch-3 expression manipulation on cell adhesion, motility and EMT in vitro revealed that the receptor could act as a tumour suppressor in NSCLC but as a tumour promoter in SCLC (28). Furthermore, while Notch-3 inhibition using siRNAs prevented cell proliferation in NSCLC cell lines, cell proliferation was stimulated in SCLC (28,29). This may have implications for the administration of Notch inhibitors in SCLC patients. Treatment of EGFR-mutated lung cancer cell lines with the EGFR tyrosine kinase inhibitor erlotinib was associated with an enrichment of the ALDH(+) stem-like cell population through EGFR-dependent activation of Notch-3, likely compromising treatment efficacy (30).
Notch implication in pharmacological interventions
In the Sage’s study, survival to cisplatin and etoposide was higher in GFP-positive than GFP-negative high tumour cell lines and correlated with an expansion of HES-1 expressing cells in longer-term protocols (4). In the patient specimens examined by the authors, high HES-1 expression was associated with poorer prognosis, supporting a key role for Notch activation in the clinical response of SCLC.
A variety of approaches are under scrutiny to target the Notch pathway in oncology (31). The administration of gamma secretase inhibitors is well under way in NSCLC. For instance combination with radiation was shown to prevent radiation-induced increase in NOTCH-3 expression and increase cellular radio-sensitivity (32). Similarly, the dual targeting of EGFR and Notch2/3 receptors with the antibody CT16 prevented acquired resistance to EGFR inhibitors and radiation through the reduction of the cancer stem cells compartment in cell line models and patient-derived xenograft tumours (33).
In a SCLC allograft model expressing low levels of HES-1, Sage et al. report that the combination of an Notch2/3 antagonist (tarextumab) with cisplatin yielder a stronger inhibition than with either treatment alone, with tarextumab inducing a delay in the development of chemoresistance. A result confirmed in a patient-derived xenograft model. This is consistent with reports that the Notch-3 receptor appears a poor activator of specific HES-1 target genes (34,35). In the human chorion carcinoma JEG cell line, transfection of the Notch-3 NICD failed to trigger HES-1 promoter activity (36,37). This data suggests a possible role for Notch-3 inhibition in the prolongation of the initial therapeutic response of SCLC.
Other emerging therapeutic approaches for SCLC include Aurora A kinase, PARP and Heat shock proteins 90 inhibition (38,39). Aurora A kinase inhibition is particularly attractive in SCLC with high MYC expression (40). But the emergence of an interaction between MYC and Notch in T-cell acute lymphoblastic leukemia (41) and in prostate cancer cells (42), warrants evaluation of the potential implication of Notch in the response of SCLC to these therapies. Similarly PARP interaction with Notch in B-cell acute lymphoblastic leukemia was proposed to impair HES-1 signalling and apoptosis induction (43). The evaluation of these interactions in SCLC is warranted.
Conclusions
The complex and heterogeneous biological landscape of SCLC can be largely attributed to the activation status of Notch signalling. Further evaluation of the pathway will likely enable the development of novel tests and therapeutic approaches with the potential to improve outcomes for patients with this aggressive disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Chen Qian (Center for Inflammation & Epigenetics, Houston Methodist Hospital Research Institute, Houston, TX, USA).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.09.22). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. [Crossref] [PubMed]
- Codony-Servat J, Verlicchi A, Rosell R. Cancer stem cells in small cell lung cancer. Transl Lung Cancer Res 2016;5:16-25. [PubMed]
- Pietanza MC, Byers LA, Minna JD, et al. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res 2015;21:2244-55. [Crossref] [PubMed]
- Lim JS, Ibaseta A, Fischer MM, et al. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature 2017;545:360-4. [Crossref] [PubMed]
- Ellisen LW, Bird J, West DC, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 1991;66:649-61. [Crossref] [PubMed]
- Previs RA CR, Harris AL, Sood AK. Molecular pathways: translational and therapeutic implications of the Notch signaling pathway in cancer. Clin Cancer Res 2015;21:955-61. [Crossref] [PubMed]
- Indraccolo S. Insights into the regulation of tumor dormancy by angiogenesis in experimental tumors. Adv Exp Med Biol 2013;734:37-52. [Crossref] [PubMed]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 2009;137:216-33. [Crossref] [PubMed]
- Shimizu K, Chiba S, Saito T, et al. Functional Diversity among Notch1, Notch2, and Notch3 Receptors. Biochemical and Biophysical Research Communications 2002;291:775-9. [Crossref] [PubMed]
- Tani S, Kurooka H, Aoki T, et al. The N- and C-terminal regions of RBP-J interact with the ankyrin repeats of Notch1 RAMIC to activate transcription. Nucleic Acids Res 2001;29:1373-80. [Crossref] [PubMed]
- Beatus P, Lundkvist J, Oberg C, et al. The origin of the ankyrin repeat region in Notch intracellular domains is critical for regulation of HES promoter activity. Mech Dev 2001;104:3-20. [Crossref] [PubMed]
- Mutvei AP, Fredlund E, Lendahl U. Frequency and distribution of Notch mutations in tumor cell lines. BMC cancer 2015;15:311. [Crossref] [PubMed]
- Bernasconi-Elias P, Hu T, Jenkins D, et al. Characterization of activating mutations of NOTCH3 in T-cell acute lymphoblastic leukemia and anti-leukemic activity of NOTCH3 inhibitory antibodies. Oncogene 2016;35:6077-86. [Crossref] [PubMed]
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. [Crossref] [PubMed]
- Ito T, Kudoh S, Ichimura T, et al. Small cell lung cancer, an epithelial to mesenchymal transition (EMT)-like cancer: significance of inactive Notch signaling and expression of achaete-scute complex homologue 1. Hum Cell 2017;30:1-10. [Crossref] [PubMed]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15:178-96. [Crossref] [PubMed]
- Hassan KA, Wang L, Korkaya H, et al. Notch pathway activity identifies cells with cancer stem cell-like properties and correlates with worse survival in lung adenocarcinoma. Clin Cancer Res 2013;19:1972-80. [Crossref] [PubMed]
- Sriuranpong V, Borges MW, Ravi RK, et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res 2001;61:3200-5. [PubMed]
- Fujino K, Motooka Y, Hassan WA, et al. Insulinoma-Associated Protein 1 Is a Crucial Regulator of Neuroendocrine Differentiation in Lung Cancer. Am J Pathol 2015;185:3164-77. [Crossref] [PubMed]
- Espinoza I, Miele L. Deadly crosstalk: Notch signaling at the intersection of EMT and cancer stem cells. Cancer Lett 2013;341:41-5. [Crossref] [PubMed]
- Hassan WA, Yoshida R, Kudoh S, et al. Notch1 controls cell invasion and metastasis in small cell lung carcinoma cell lines. Lung Cancer 2014;86:304-10. [Crossref] [PubMed]
- Poirier JT, Dobromilskaya I, Moriarty WF, et al. Selective tropism of Seneca Valley virus for variant subtype small cell lung cancer. J Natl Cancer Inst 2013;105:1059-65. [Crossref] [PubMed]
- Augustyn A, Borromeo M, Wang T, et al. ASCL1 is a lineage oncogene providing therapeutic targets for high-grade neuroendocrine lung cancers. Proc Natl Acad Sci U S A 2014;111:14788-93. [Crossref] [PubMed]
- Borromeo MD, Savage TK, Kollipara RK, et al. ASCL1 and NEUROD1 Reveal Heterogeneity in Pulmonary Neuroendocrine Tumors and Regulate Distinct Genetic Programs. Cell Rep 2016;16:1259-72. [Crossref] [PubMed]
- Saunders LR, Bankovich AJ, Anderson WC, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 2015;7:302ra136 [Crossref] [PubMed]
- Rudin CM, Pietanza MC, Bauer TM, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 2017;18:42-51. [Crossref] [PubMed]
- Yuan X, Wu H, Xu H, et al. Meta-analysis reveals the correlation of Notch signaling with non-small cell lung cancer progression and prognosis. Sci Rep 2015;5:10338. [Crossref] [PubMed]
- Hassan WA, Yoshida R, Kudoh S, et al. Evaluation of role of Notch3 signaling pathway in human lung cancer cells. Journal of Cancer Research and Clinical Oncology 2016;142:981-93. [Crossref] [PubMed]
- Shi C, Qian J, Ma M, et al. Notch 3 protein, not its gene polymorphism, is associated with the chemotherapy response and prognosis of advanced NSCLC patients. Cell Physiol Biochem 2014;34:743-52. [Crossref] [PubMed]
- Arasada RR, Amann JM, Rahman MA, et al. EGFR blockade enriches for lung cancer stem-like cells through Notch3-dependent signaling. Cancer Res 2014;74:5572-84. [Crossref] [PubMed]
- Yuan X, Wu H, Xu H, et al. Notch signaling: an emerging therapeutic target for cancer treatment. Cancer Lett 2015;369:20-7. [Crossref] [PubMed]
- Ikezawa Y, Sakakibara-Konishi J, Mizugaki H, et al. Inhibition of Notch and HIF enhances the antitumor effect of radiation in Notch expressing lung cancer. Int J Clin Oncol 2017;22:59-69. [Crossref] [PubMed]
- Hu S, Fu W, Li T, et al. Antagonism of EGFR and Notch limits resistance to EGFR inhibitors and radiation by decreasing tumor-initiating cell frequency. Sci Transl Med 2017;9. [PubMed]
- Kageyama R, Ohtsuka T. The Notch-Hes pathway in mammalian neural development. Cell Research 1999;9:179-88. [Crossref] [PubMed]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 2006;7:678-89. [Crossref] [PubMed]
- Beatus P, Lundkvist J, Oberg C, et al. The notch 3 intracellular domain represses notch 1-mediated activation through Hairy/Enhancer of split (HES) promoters. Development 1999;126:3925-35. [PubMed]
- Bellavia D, Checquolo S, Campese AF, et al. Notch3: from subtle structural differences to functional diversity. Oncogene 2008;27:5092-8. [Crossref] [PubMed]
- Seeber A, Leitner C, Philipp-Abbrederis K, et al. What's new in small cell lung cancer - extensive disease? An overview on advances of systemic treatment in 2016. Future Oncol 2017;13:1427-35. [Crossref] [PubMed]
- Subramaniam DS, Warner EA, Giaccone G. Ganetespib for small cell lung cancer. Expert Opin Investig Drugs 2017;26:103-8. [Crossref] [PubMed]
- Mollaoglu G, Guthrie MR, Bohm S, et al. MYC Drives Progression of Small Cell Lung Cancer to a Variant Neuroendocrine Subtype with Vulnerability to Aurora Kinase Inhibition. Cancer Cell 2017;31:270-85. [Crossref] [PubMed]
- Sanchez-Martin M, Ferrando A. The NOTCH1-MYC highway toward T-cell acute lymphoblastic leukemia. Blood 2017;129:1124-33. [Crossref] [PubMed]
- Frank SB, Berger PL, Ljungman M, et al. Human prostate luminal cell differentiation requires NOTCH3 induction by p38-MAPK and MYC. J Cell Sci 2017;130:1952-64. [Crossref] [PubMed]
- Kannan S, Fang W, Song G, et al. Notch/HES1-mediated PARP1 activation: a cell type-specific mechanism for tumor suppression. Blood 2011;117:2891-900. [Crossref] [PubMed]


