
HIF-1α and VEGF levels for monitoring hepatocellular carcinoma treatment response to transcatheter arterial chemoembolization
Introduction
Hepatocellular carcinoma (HCC) is one of the most common human malignancies. Because of the insidious pathogenesis of HCC, most patients with HCC are already in advanced stages when seeking treatment and have lost the opportunity for surgical resection. Transcatheter arterial chemoembolization (TACE) is the most common treatment option in HCC patients who cannot receive potentially curative therapies such as resection and transplantation. Chemoembolization for HCC has been proven to be useful in local tumor control, to prevent tumor progression, prolong patients’ life and control patient symptoms (1-6). However, the long-term efficacy of TACE therapy against HCC is far from ideal. Tumor angiogenesis of the residual disease is one of the important factors that affect the efficacy of TACE in the treatment of HCC (7-10). There is ample evidence that tumor angiogenesis is the pathological basis of and a necessary condition for solid tumor growth and metastasis (11-13). Among the many regulatory factors in tumor angiogenesis, hypoxia-inducible factor-1 alpha (HIF-lα) and vascular endothelial growth factor (VEGF) are the key inducing factors of tumor angiogenesis and are involved in all stages of the process (14-18). Some researchers have studied the changes in VEGF expression that occurred in patients with HCC after TACE (19-26); however, few studies addressed the dynamic changes in the serum levels of HIF-1α that occur in patients with HCC after TACE. This prospective study aimed to assess the dynamic changes in the serum levels of both HIF-1α and VEGF in HCC patients after TACE and to determine whether the levels of the above-listed factors change in response to TACE. In addition, the correlation between HIF-1α and VEGF was also analyzed.
Methods
Clinical data
This study was approved by our institutional review board, and patient informed consent was obtained. A total of 41 HCC patients were selected, including 36 males and 5 females. All cases were confirmed by percutaneous biopsy or typical imaging findings (dynamic enhanced computed tomography or magnetic resonance imaging) for HCC associated with a pathological increase of serum alpha-fetoprotein (AFP) levels. The ages of the patients ranged from 26 to 69 years (mean age 52.6±11.1 years). All of the patients in this group cannot receive surgical resection. None of the patients received any other anti-tumor therapy prior to the TACE procedure. Four weeks after TACE treatments, the overall tumor response was evaluated according to the modified Response Evaluation Criteria in Solid Tumors criteria (27). Patients with a complete response (CR) or partial response (PR) comprised the responding group, whereas those with stable disease (SD) or progressive disease (PD) comprised the non-responding group.
TACE procedure
In 41 patients, the chemoembolization procedure was conducted according to the method reported in our previous study (28). After diagnostic digital subtraction angiography and superselective catheterization, TACE was performed by administration of 5-fluorouracil (1,000 to 1,500 mg) and hydroxycamptothecine (30 to 40 mg), followed by lipiodol (3 to 20 mL) with adriamycin (40 to 50 mg) emulsion and gelfoam particles. The volume of embolus was determined by taking into account the hepatic functional indices, the diameter of the lesion and the vascularity of the tumor.
Biomarker assessment
Fasting peripheral venous blood samples (4 mL) were collected from each patient in the morning 1 day prior to the TACE procedure, as well as 1, 7 and 28 days after the TACE treatment. The blood samples were placed in sterile tubes and allowed to stand for 30–60 min. The blood samples were then centrifuged at 3,000 r/min for 15 min. After centrifugation, serum was collected and stored in a −80 °C freezer for future assays. Serum HIF-1α and VEGF levels were determined using enzyme-linked immunosorbent assays (ELISAs) [human total HIF-lα Elisa kit (DYC1935-2), human VEGF Elisa kit (DVE00); R & D Systems, Inc., Minneapolis, MN 55413, USA].
Statistical methods
Serum HIF-1α and VEGF levels were statistically analyzed using SPSS 21.0 statistical software (SPSS Inc., Chicago, Illinois, USA). In the statistical analysis, values of serum HIF-1α and VEGF levels were expressed as median, first and third quartiles. The differences in serum HIF-1α and VEGF levels before and after TACE therapy were subjected to analysis of nonparametric test, while correlations were examined using Pearson’s correlation analysis. Receiver-operating characteristic (ROC) curve was applied to analyze the evaluation value of the factors for the response of TACE on the treatment of HCC. P<0.05 was considered statistically significant.
Results
Preoperative AFP was positive in 31 subjects (75.6%) and negative in 10 subjects (24.4%). HBsAg (+) occurred in 38 subjects (92.7%) and HBsAg (−) occurred in 3 subjects (7.3%). Liver function in the HCC patients was evaluated using the Child-Pugh grading scale. Twenty-nine patients (70.7%) were classified as Child-Pugh grade A, while 12 patients (29.3%) were classified as Child-Pugh grade B. All patients’ diseases were staged using Barcelona Clinic Liver Cancer’s (BCLC) scheme: 32 patients (78.0%) had stage B, and 9 patients (22.0%) had stage C tumors. Nine patients (22.0%) were confirmed with presence of portal vein invasion, and 5 patients (12.2%) were confirmed with presence of metastasis. Sixteen subjects (39.0%) had an intact capsule and 25 subjects (61.0%) had no capsule or infiltration. The maximum tumor diameters ranged from 4.1 to 13.2 cm (mean 7.2±3.7 cm).
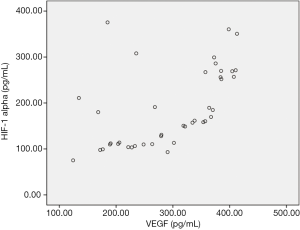
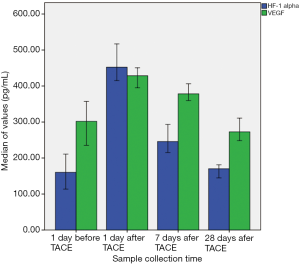
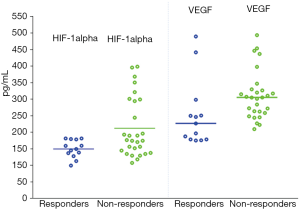
After treatment, PR was achieved in 13 patients (31.7%), SD was achieved in 18 patients (43.9%), and PD was achieved in 10 patients (24.4%), none of the patients achieved CR. The serum levels of HIF-1α correlated positively with the serum levels of VEGF 1 day before TACE (r=0.546, P=0.000) (Figure 1). The levels of HIF-1α and VEGF 1 day before, and 1, 7 and 28 days after TACE were significantly different, respectively (χ2=90.688, P=0.000 and χ2=45.585, P=0.000). The levels of the HIF-1α and VEGF increased markedly on day 1 and 7 after TACE and recovered to the pre-TACE level on day 28 after TACE (Table 1, Figure 2). The levels of serum VEGF in responder group 28 days after TACE were significantly lower than those in non-responder group (Z=2.774, P=0.006), but the difference of HIF-1α levels between the two groups was not significant (Z=1.905, P=0.057) (Figure 3, Table 2). ROC curve analysis indicated that the sensitivity and specificity were 76.9% and 78.6%, when the threshold value was set at VEGF =254.5 pg/mL for predicting the response of TACE in patients with HCC; the corresponding area under the curve (AUC) was 0.772, respectively.
Full table

Full table
Discussion
Tumor angiogenesis is closely related to the development, progression and metastasis of solid tumors. Among the numerous angiogenesis-related factors, HIF-1α and VEGF have attracted considerable attention due to their key roles in promoting angiogenesis. HIF-1α is overexpressed under hypoxic conditions, which induces the transcription of the VEGF gene and upregulates the expression of VEGF and its receptor, thereby promoting tumor angiogenesis (29-31). The cognate DNA recognition site of HIF-1α is hypoxia response element (HRE). The binding of HIF-1α to HRE in the VEGF promoter is a predominant enhancer of VEGF production (29,30). VEGF was reported to promote angiogenesis by inducing migration and proliferation of endothelial cells. VEGF protein binds to VEGF receptors on endothelial cells, and these mediate its physiological functions (29,30).
In recent years, some scholars investigated the post-TACE changes in serum VEGF levels that occur in patients with HCC and explored the significance of those changes in evaluating the response to TACE therapy (19-26). Li et al. (19) measured plasma VEGF levels in 45 HCC patients prior to TACE therapy and 1, 3, 7 days and 1 month after TACE therapy. The results showed that the plasma VEGF level was significantly higher in patients with HCC than in patients with benign lesions and healthy volunteers. In addition, plasma VEGF levels were drastically increased in HCC patients after TACE therapy. Plasma VEGF levels reached a peak value on the first day after therapy and then decreased gradually. Moreover, VEGF levels were related to therapeutic efficacy; TACE yielded better therapeutic efficacy in patients with low pre-TACE levels of VEGF, whereas TACE was less effective in patients with high pre-TACE levels of VEGF. Many other studies revealed similar dynamic changes in VEGF levels in patients with HCC after TACE therapy (20-26).
However, a number of studies yielded results that are not entirely consistent with the above findings (32,33). For example, Suzuki et al. (32) reported that in a group of 38 patients with HCC, VEGF levels did not increase until 1 week after TACE therapy. Chao et al. (33) examined post-TACE VEGF levels in 41 patients with HCC. Those authors found that VEGF levels increased even more slowly and reached a peak value 14 days after TACE therapy.
To date, few studies have focused on the dynamic changes in serum HIF-1α levels that occur in patients with HCC after TACE therapy, and the use of HIF-1α and VEGF levels to monitor the treatment response to TACE in HCC. Jia et al. (34) performed ELISA to examine the changes in the expression of HIF-1α and VEGF in 40 patients with primary liver cancer (PLC) before and after TACE therapy. The results showed that serum HIF-1α and VEGF levels in patients with PLC rose significantly to a peak at 1 day after TACE therapy. Serum HIF-1α and VEGF levels were then markedly reduced 1 week after TACE therapy and remained higher than the pre-TACE levels. The present study simultaneously explored the dynamic changes in serum HIF-1α and VEGF levels that occur in HCC patients who received TACE therapy. The results showed that serum HIF-1α and VEGF levels in HCC patients both rose to peak values at 1 day after TACE therapy. The serum HIF-1α and VEGF levels then decreased gradually and finally returned to pre-TACE levels 28 days after TACE therapy. The levels of VEGF in responding group 28 days after TACE were significantly lower than those in non-responding group, but the difference of HIF-1α levels between the two groups was not significant. In addition, the values of responders and non-responders overlap.
The present study has the following limitations: first, the sample size was rather small; second, the serum levels of HIF-1α and VEGF in patients with HCC after TACE will changes dynamically at different times; however, in this experiment, the serum levels of HIF-1α and VEGF only detected 1, 7 and 28 days after TACE therapy, with no analysis of serum levels of HIF-1α and VEGF during other periods. Another limitation is that the relationship between the observed changes in HIF-1α and VEGF levels after TACE therapy and prognosis was not investigated in the HCC patients. In further studies, an increased number of medical cases should be examined and more time points should be selected to analyze serum HIF-1α and VEGF levels. In addition, the prognosis of the patients should be followed up. Hence, a deep understanding of the dynamic changes in HIF-1α and VEGF levels that occur in patients with HCC after TACE therapy and the relationship between those changes and prognosis could be achieved.
In summary, our results indicated that in patients with HCC, serum levels of HIF-1α and VEGF demonstrated dynamic changes after TACE therapy, first increasing and then decreasing. However, HIF-1α and VEGF may be insufficient for predicting tumor response to TACE treatment. As previous studies have shown that imaging examination play an important role in evaluating tumor treatment response (28,35-42). Therefore, for a specific individual, serum HIF-1α and VEGF changes combined with imaging changes are more conducive to accurately evaluate the TACE treatment response.
Acknowledgments
Funding: This work was supported by the Projects of Department of Science and Technology of Sichuan Province (2016JY0105).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yì-Xiáng J. Wáng, Yong Wang) for the series “Translational Imaging in Cancer Patient Care” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.08.32). The series “Translational Imaging in Cancer Patient Care” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Affiliated Hospital of North Sichuan Medical College (No. ER2017A0213), and patient informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164-71. [Crossref] [PubMed]
- Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9. [Crossref] [PubMed]
- Wáng YX, De Baere T, Idée JM, et al. Transcatheter embolization therapy in liver cancer: an update of clinical evidences. Chin J Cancer Res 2015;27:96-121. [PubMed]
- Song DS, Nam SW, Bae SH, et al. Outcome of transarterial chemoembolization-based multi-modal treatment in patients with unresectable hepatocellular carcinoma. World J Gastroenterol 2015;21:2395-404. [Crossref] [PubMed]
- Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J Gastroenterol 2015;21:10327-35. [Crossref] [PubMed]
- Molinari M, Kachura JR, Dixon E, et al. Transarterial chemoembolisation for advanced hepatocellular carcinoma: results from a North American cancer centre. Clin Oncol (R Coll Radiol) 2006;18:684-92. [Crossref] [PubMed]
- Omyła-Staszewska J, Deptała A. Effective therapeutic management of hepatocellular carcinoma - on the basis of a clinical case. Contemp Oncol (Pozn) 2012;16:60-3. [PubMed]
- Gupta S, Kobayashi S, Phongkitkarun S, et al. Effect of transcatheter hepatic arterial embolization on angiogenesis in an animal model. Invest Radiol 2006;41:516-21. [Crossref] [PubMed]
- Guo WJ, Li J, Chen Z, et al. Transient increased expression of VEGF and MMP-1 in a rat liver tumor model after hepatic arterial occlusion. Hepatogastroenterology 2004;51:381-6. [PubMed]
- Unruh A, Ressel A, Mohamed HG, et al. The hypoxia-inducible factor-1 alpha is a negative factor for tumor therapy. Oncogene 2003;22:3213-20. [Crossref] [PubMed]
- Schwab LP, Peacock DL, Majumdar D, et al. Hypoxia-inducible factor 1α promotes primary tumor growth and tumor-initiating cell activity in breast cancer. Breast Cancer Res 2012;14:R6. [Crossref] [PubMed]
- Liao D, Corle C, Seagroves TN, et al. Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res 2007;67:563-72. [Crossref] [PubMed]
- Chang Q, Qin R, Huang T, et al. Effect of antisense hypoxia-inducible factor 1alpha on progression, metastasis, and chemosensitivity of pancreatic cancer. Pancreas 2006;32:297-305. [Crossref] [PubMed]
- Dang DT, Chen F, Gardner LB, et al. Hypoxia-inducible factor-1alpha promotes nonhypoxia-mediated proliferation in colon cancer cells and xenografts. Cancer Res 2006;66:1684-936. [Crossref] [PubMed]
- Kim JW, Evans C, Weidemann A, et al. Loss of fibroblast HIF-1α accelerates tumorigenesis. Cancer Res 2012;72:3187-95. [Crossref] [PubMed]
- Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature 2000;407:242-8. [Crossref] [PubMed]
- Yamaguchi R, Yano H, Nakashima Y, et al. Expression and localization of vascular endothelial growth factor receptors in human hepatocellular carcinoma and non-HCC tissues. Oncol Rep 2000;7:725-9. [PubMed]
- Liu K, Min XL, Peng J, et al. The Changes of HIF-1α and VEGF Expression After TACE in Patients With Hepatocellular Carcinoma. J Clin Med Res 2016;8:297-302. [Crossref] [PubMed]
- Li X, Feng GS, Zheng CS, et al. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol 2004;10:2878-82. [Crossref] [PubMed]
- Ranieri G, Ammendola M, Marech I, et al. Vascular endothelial growth factor and tryptase changes after chemoembolization in hepatocarcinoma patients. World J Gastroenterol 2015;21:6018-25. [Crossref] [PubMed]
- Jia ZZ, Huang YQ, Feng YL, et al. Correlations between serum hypoxia inducible factor-1α, vascular endothelial growth factor and computed tomography perfusion imaging at pre-and post-TACE in patients with primary hepatic carcinoma. Zhonghua Yi Xue Za Zhi 2013;93:1472-5. [PubMed]
- Guo JH, Zhu X, Li XT, et al. Impact of serum vascular endothelial growth factor on prognosis in patients with unresectable hepatocellular carcinoma after transarterial chemoembolization. Chin J Cancer Res 2012;24:36-43. [Crossref] [PubMed]
- Bao Y, Feng WM, Tang CW, et al. Endostatin inhibits angiogenesis in hepatocellular carcinoma after transarterial chemoembolization. Hepatogastroenterology 2012;59:1566-8. [PubMed]
- Hsieh MY, Lin ZY, Chuang WL. Serial serum VEGF-A, angiopoietin-2, and endostatin measurements in cirrhotic patients with hepatocellular carcinoma treated by transcatheter arterial chemoembolization. Kaohsiung J Med Sci 2011;27:314-22. [Crossref] [PubMed]
- Shim JH, Park JW, Kim JH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci 2008;99:2037-44. [PubMed]
- Liu J, Yi J. Relationship between the changes of VEGF level and dendritic cells in peripheral blood of patients with hepatocellular carcinoma after transcatheter arterial chemoembolization. J Huazhong Univ Sci Technolog Med Sci 2007;27:58-60. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Yang L, Zhang XM, Tan BX, et al. Computed tomographic perfusion imaging for the therapeutic response of chemoembolization for hepatocellular carcinoma. J Comput Assist Tomogr 2012;36:226-30. [Crossref] [PubMed]
- Tsuzuki Y, Fukumura D, Oosthuyse B, et al. Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1alpha--> hypoxia response element--> VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Res 2000;60:6248-52. [PubMed]
- Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol 1999;15:551-78. [Crossref] [PubMed]
- Choi KS, Bae MK, Jeong JW, et al. Hypoxia-induced angiogenesis during carcinogenesis. J Biochem Mol Biol 2003;36:120-7. [PubMed]
- Suzuki H, Mori M, Kawaguchi C, et al. Serum vascular endothelial growth factor in the course of transcatheter arterial embolization of hepatocellular carcinoma. Int J Oncol 1999;14:1087-90. [PubMed]
- Chao Y, Wu CY, Kuo CY, et al. Cytokines are associated with postembolization fever and survival in hepatocellular carcinoma patients receiving transcatheter arterial chemoembolization. Hepatol Int 2013;7:883-92. [Crossref] [PubMed]
- Jia ZZ, Jiang GM, Feng YL. Serum HIF-1alpha and VEGF levels pre- and post-TACE in patients with primary liver cancer. Chin Med Sci J 2011;26:158-62. [Crossref] [PubMed]
- Loffroy R, Favelier S, Cherblanc V, et al. C-arm dual-phase cone-beam CT: a revolutionary real-time imaging modality to assess drug-eluting beads TACE success in liver cancer patients. Quant Imaging Med Surg 2013;3:196-9. [PubMed]
- Yuan J, Lo G, King AD. Functional magnetic resonance imaging techniques and their development for radiation therapy planning and monitoring in the head and neck cancers. Quant Imaging Med Surg 2016;6:430-48. [Crossref] [PubMed]
- Yang K, Zhang XM, Yang L, et al. Advanced imaging techniques in the therapeutic response of transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol 2016;22:4835-47. [Crossref] [PubMed]
- Takayasu K, Arii S, Matsuo N, et al. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol 2000;175:699-704. [Crossref] [PubMed]
- Xu X, Luo L, Chen J, et al. Acoustic radiation force impulse elastography for efficacy evaluation after hepatocellular carcinoma radiofrequency ablation: a comparative study with contrast-enhanced ultrasound. Biomed Res Int 2014;2014:901642 [PubMed]
- Wáng YX, Idée JM. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant Imaging Med Surg 2017;7:88-122. [Crossref] [PubMed]
- Wu XM, Wang JF, Ji JS, et al. Evaluation of efficacy of transcatheter arterial chemoembolization for hepatocellular carcinoma using magnetic resonance diffusion-weighted imaging. Onco Targets Ther 2017;10:1637-43. [Crossref] [PubMed]
- Li YT, Cercueil JP, Yuan J, et al. Liver intravoxel incoherent motion (IVIM) magnetic resonance imaging: a comprehensive review of published data on normal values and applications for fibrosis and tumor evaluation. Quant Imaging Med Surg 2017;7:59-78. [Crossref] [PubMed]


