
Role of p53 in regulating tissue response to radiation by mechanisms independent of apoptosis
Introduction
The tumor suppressor p53 is a master regulator of cellular response to radiation (1-3). p53 is a multifunctional transcription factor containing two transcriptional activation domains that can independently enhance transcription of downstream target genes, and a DNA binding domain responsible for sequence-specific binding of p53 to its response elements (4). Upon radiation exposure, activation of the DNA damage response increases the level of p53 protein in cells primarily by promoting protein translation (5) and inhibiting protein degradation (6). Accumulation of p53 protein in the nucleus induces a variety of downstream signaling pathways that mediate cellular response to stress (7,8). Activation of p53-mediated signaling can cause cell cycle arrest and facilitate DNA repair, which promote cell survival, or induce the intrinsic pathway of apoptosis and cell senescence, which augment cell death. Therefore, p53 plays a crucial role in controlling cellular fate after irradiation.
Several factors influence the p53 response following irradiation, including the intensity of stress, the presence of co-factors that interact with p53, and DNA binding cooperativity of p53 (7,9). Additionally, it has been shown in mice that total-body irradiation induces p53 and its downstream signaling in vivo in a tissue-dependent manner (8,10,11). For example, activation of p53 results in dramatically increased pre-mitotic apoptosis in tissues that have a rapid turnover rate such as the hematopoietic system and the gastrointestinal (GI) epithelium. To the contrary, in tissues with a slower turnover rate, such as the myocardium, accumulation of p53 following radiation does not cause a significant increase in pre-mitotic apoptosis, but instead induces genes that control cell-cycle checkpoints such as the cyclin-dependent kinase inhibitor p21. Moreover, recent studies in the hematopoietic system suggest that p53 activation results in a distinct response in stem cells versus progenitors (12,13). Thus, these findings reveal the diversity of p53 response following radiation in vivo and underscore the significance of dissecting the mechanisms by which p53 controls radiation response in a cell-type specific manner.
To dissect the role of p53 in response to radiation in vivo, several groups have performed mechanistic studies using mice that either lack p53 or its transcriptional targets, or using the Cre-loxP system (14) to spatially and/or temporally disrupt p53 in mice. In this review, we summarize recent advances in understanding the role of p53-mediated signaling in regulating radiation response through mechanisms that are independent of apoptosis in the hematopoietic system, the GI epithelium and vascular endothelial cells.
Role of p53 in controlling radiation response in hematopoietic stem and progenitor cells
Radiation causes acute and long-term toxicity in the hematopoietic compartment
The hematopoietic system is very sensitive to radiation. After irradiation, a rapid increase in pre-mitotic apoptosis ablates hematopoietic progenitor cells and more differentiated hematopoietic cells (15), leading to acute hematological radiation toxicity due to the short-term loss of functioning blood cells (16,17). The overall response of the hematopoietic compartment is mediated by apoptosis in the acute phase following radiation exposure, coupled with long-term defects in hematopoietic stem cells (HSCs) after the recovery phase (18). The reduction in fitness of irradiated HSCs, which is associated with cell senescence (18-20), has been demonstrated using competitive repopulation assays. HSCs from mice that are exposed to lethal or sub-lethal doses of total-body irradiation have a dramatic decrease in long-term engraftment in the bone marrow compared to unirradiated HSCs (19,21,22). Collectively, these results demonstrate that radiation exposure causes short-term and long-term damage to the hematopoietic compartment. While acute hematological radiation toxicity is primarily attributed to apoptosis, chronic hematological toxicity is at least partially caused by apoptosis-independent mechanisms.
Loss of p53 ameliorates acute hematological radiation toxicity by blocking apoptosis
Radiation induces the DNA damage response to active p53 in hematopoietic stem cells and progenitors (HSPCs) (12,13,23). However, the response of p53 to radiation varies between stem and progenitor cells. While p53 activation engages radiation-induced apoptosis in hematopoietic progenitors, activation of p53 in short-term HSCs does not induce the intrinsic pathway of apoptosis. Moreover, radiation does not induce detectable levels of phosphorylated p53 in long-term HSCs (13). It has been shown that p53 is necessary to promote radiation-induced apoptosis in hematopoietic cells because deletion of p53 in mice (p53-/- mice) dramatically abrogates radiation-induced apoptosis and ameliorates acute hematological radiation toxicity (24-26). The essential role of p53-mediated apoptosis in acute hematopoietic toxicity is further demonstrated using mice that lack PUMA (p53 upregulated modulator of apoptosis), a transcriptional target of p53 that activates the intrinsic pathway of apoptosis (27,28). Compared to PUMA+/+ mice, both PUMA+/- and PUMA-/- mice are resistant to acute hematological radiation toxicity due to a dramatic decrease in apoptosis in hematopoietic progenitor cells (29,30).
Loss of p53 improves long-term engraftment potential of irradiated HSCs
It has been challenging to study long-term effects of radiation on the hematopoietic system in p53-/- mice due to the extremely high penetrance of spontaneous lymphomas (31,32). Recent studies investigated how p53 controls long-term fitness of HSPCs after total-body irradiation using bone marrow chimeric mice that harbor only a small portion of p53-deficient cells. Marusyk et al. generated bone marrow chimera containing approximately 15% GFP-tagged p53-/- cells. After 2.5 Gy total-body irradiation, the percentage of p53-/- peripheral blood cells in chimeric mice increased significantly compared to unirradiated controls, indicating that p53 disruption confers radioresistance and facilitates clonal expansion of HSPCs (33). These results indicate that deletion of p53 in HSPCs improves their clonogenic capacity after irradiation over HSPCs with wild-type p53 because radiation-induced apoptosis is blocked in progenitors and because stem/progenitor cells are protected from radiation-induced loss of fitness.
To address a similar question, Bondar et al. generated a novel conditional allele in which a GFP-tagged oncogenic p53 mutant R172H (mp53) can be temporally induced in the whole animal by tamoxifen. Injection with a single dose of tamoxifen created bone marrow chimeric mice that contain a small portion (<5%) of mp53 cells in peripheral blood. After exposure to 2.5 Gy total-body irradiation, the percentage of mp53 blood cells increased dramatically and even persisted 200 days after irradiation, demonstrating the expansion of mp53 cells in the long-term HSC pool (34). Interestingly, induction of mp53 either two days before or 7 days after irradiation, when the DNA damage response is diminished, significantly increased the percentage of mp53 cells in hematopoietic cells. These results indicate that in addition to the DNA damage response, stress stimuli that are secondary to radiation, such as increased reactive oxygen species (22,35,36), impair the fitness of HSPCs that have functional p53. In addition, deletion of cyclin-dependent kinase inhibitor 2A gene, which transcribes both p16INK4a and p19ARF in mice (37), partially improves the clonogenic capacity of p53-wild type HSPCs after irradiation, suggesting that senescence contributes to radiation-induced defects in HSPCs. Together, these findings indicate that a permanent change in p53 activity improves the fitness of HSPCs by blocking acute apoptosis, which is induced by the DNA damage response and by suppressing delayed senescence, which may be induced by an altered microenvironment after irradiation.
Deletion of p21 allows human fibroblasts to bypass senescence in response to DNA damage (38), suggesting that p21 may also play a role in regulating radiation-induced senescence in vivo. However, different groups have shown that loss of p21 exacerbates defects in long-term engraftment potential of irradiated HSCs (13,39). These data indicate that p21 is necessary to protect HSCs against radiation. Interestingly, Insinga and colleagues found that radiation upregulated p21 in short-term HSCs and long-term HSCs via p53-dependent and p53-independent mechanisms, respectively (13). In addition, p21 protein actually suppressed radiation-induced p53 activation in long-term HSCs because deletion of p21 in long-term HSCs increased phosphorylated of p53 protein and apoptosis after irradiation. These results indicate that p21 regulates the response to radiation in HSCs through mechanisms that either dependent or independent of p53. Further studies are warranted to understand the mechanisms by which radiation induces p21 in a p53-independent manner and how p21 suppresses p53 activation in long-term HSCs after irradiation.
Summary
A reduced level of p53 in the hematopoietic compartment promotes radiation resistance, making p53 a promising target for preventing the acute hematopoietic syndrome and/or residual bone marrow toxicity. However, the manner in which p21 cooperates with p53 to regulate radiation response in short-term and long-term HSCs remains to be better understood (Figure 1A). In addition, there is a concern about the long-term consequences of reducing p53 (such as thymic lymphoma) during radiation because of its function as a tumor suppressor (46). Therefore, further studies are warranted to evaluate the effect of temporarily blocking p53 during irradiation on radiation toxicity of the hematopoietic system and radiation-induced cancer.
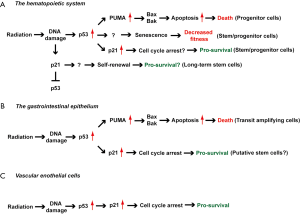
Role of p53-mediated signaling in the radiation-induced GI syndrome
Loss of p53 sensitizes mice to the radiation-induced GI syndrome
Exposure of the GI tract to radiation causes acute GI toxicity or the GI syndrome (47). The GI syndrome is caused by destruction of the GI epithelium, which leads to infection and loss of fluid and electrolytes (48,49). The integrity of the small intestine is dependent on a constant state of renewal driven by the stem cells residing in the crypts. Radiation impairs the regeneration of intestinal epithelium predominantly by inducing cell death in crypt epithelial cells (50,51). Crypt epithelial cells are highly sensitive to radiation-induced pre-mitotic apoptosis, which occurs within a few hours after irradiation (50). It has been shown that p53-mediated signaling plays a pivotal role in promoting pre-mitotic apoptosis of crypt epithelial cells because crypt epithelial cells in p53-/- mice are dramatically resistant to radiation-induced apoptosis that occurs 4 to 6 hours after irradiation (41,42).
While crypt epithelial cells in p53-/- mice are resistant to radiation-induced apoptosis, p53-/- mice are surprisingly more sensitive to the radiation-induced GI syndrome (26). Detailed time course studies after radiation exposure show that p53-/- mice have a delayed onset of cell death in crypt epithelial cells that occurs approximately 24 hours after irradiation (52). Thus, it is possible that loss of p53 sensitizes crypt epithelial cells to mitotic death, which results from aberrant segregation of the genomic DNA during mitosis (53,54). Mitotic catastrophe is frequently observed in cells that have a defect in cell cycle checkpoints (54). Indeed, in the first 24 hours after irradiation, crypt epithelial cells of p53+/+ mice show a decrease in cell proliferation; however, crypt epithelial cells of p53-/- mice have a defect in cell cycle arrest and continue to proliferate (26,52). Collectively, these results indicate a diverse role of p53 in regulating the survival of crypt epithelial cells.
The intrinsic pathway of apoptosis in GI epithelial cells does not contribute to the radiation-induced GI syndrome
To specifically investigate the role of the intrinsic pathway of apoptosis in the radiation-induced GI syndrome, we utilized the Cre-loxP system to generate mice with GI epithelium-specific deletion of Bak (Bcl-2 homologous antagonist killer) and Bax (Bcl-2 associated X protein) (VillinCre; BaxFL/-; Bak-/-) (40). Bak and Bax are key pro-apoptotic proteins that govern mitochondrial outer membrane permeabilization to irreversibly initiate the intrinsic pathway of apoptosis (55,56). Remarkably, deletion of both Bak and Bax in the GI epithelium decreased radiation-induced apoptosis in crypt epithelial cells, but it did not protect mice from the radiation-induced GI syndrome (40). In contrast, specific deletion of p53 in the GI epithelium significantly exacerbated the GI syndrome, which recapitulates the phenotype that was observed in p53-/- mice (40). Moreover, deletion of Bak and Bax did not rescue the radiation sensitivity of the GI tract resulting from loss of p53. Together, these results demonstrate that (I) survival from the GI syndrome is not increased by blocking the intrinsic pathway of apoptosis in GI epithelial cells and (II) loss of p53 sensitizes GI epithelial cells to radiation through mechanisms that are independent of pre-mitotic apoptosis.
Loss of PUMA protects mice from the GI syndrome via the cyclin-dependent kinase inhibitor p21
Other groups also investigated the role of p53-mediated apoptosis in controlling the radiation-induced GI syndrome using mice with whole animal knockout of PUMA (43). Remarkably, PUMA-/- mice not only showed a defect in radiation-induced apoptosis in the crypts, but also had improved survival from the GI syndrome (43). These results suggest that blocking PUMA-mediated apoptosis may protect mice from the GI syndrome, which appears to contradict the results using mice with GI epithelium-specific deletion of Bak and Bax. However, because PUMA functions upstream of Bak and Bax to initiate pre-mitotic apoptosis (10), it is possible that deletion of PUMA protects mice from the GI syndrome through mechanisms that are independent of its role in regulating apoptosis (57). Indeed, through mechanisms that are not well understood, the GI epithelium of PUMA-/- mice has elevated levels of the cyclin-dependent kinase inhibitor p21 (43,58). Thus, up-regulation of p21 may function in the resistance to the radiation-induced GI syndrome resulting from deletion of PUMA.
The role of p21 in the radiation-induced GI syndrome has been examined in several studies using p21-/- mice. The results from these studies demonstrate that p21-/- mice are more sensitive to the radiation-induced GI syndrome than mice retaining functional p21 (26,40,58), indicating that p21-mediates signaling is necessary to prevent mice from developing the GI syndrome. To elucidate whether p21 is necessary for the resistance of PUMA-/- mice to the GI syndrome, Leibowitz et al. investigated the radiation-induced GI syndrome in p53-/-, PUMA-/-, p21-/- and PUMA-/-; p21-/- (double knockout) mice (58). Their results showed that PUMA-/-; p21-/- mice developed the radiation-induced GI syndrome significantly faster than PUMA-/- mice, indicating that p21 is also necessary to confer resistance to the GI syndrome in PUMA-/- mice. Remarkably, although p53-/-, p21-/- and PUMA-/-; p21-/- mice were more sensitive to the GI syndrome compared to wild-type mice; these mice all had a significantly higher number of regenerated crypts in the small intestine 72 hours after irradiation, which is likely due to compromised cell cycle arrest (59,60). Defects in cell cycle arrest in these mice elicit a higher percentage of crypt cells that undergo aberrant mitosis or mitotic catastrophe, which results in delayed cell death after irradiation (58). Consistent with this model, we found that “super p53 mice”, which harbor an extra copy of p53 (61), are more resistant to the radiation-induced GI syndrome via a mechanism that is also dependent on p21 (14,62). Taken together, these results demonstrate a pivotal role of the p53/p21 axis in protecting mice against the radiation-induced GI radiation syndrome by preventing crypt cells from premature mitotic entry after irradiation.
Mitotic catastrophe contributes to cell death in intestinal stem cells after irradiation
The increased sensitivity of p53-/- and p21-/- mice to GI syndrome reveals that certain types of intestinal stem cells (ISCs) (63) essential to regenerate the GI epithelium after radiation injury may be killed through mitotic catastrophe. Indeed, in the small intestine of wild-type mice, radiation not only induces pre-mitotic apoptosis, but also causes aberrant mitosis and mitotic death in crypt epithelial cells (40,64). To elucidate the mechanisms by which ISCs die from radiation, a recent study (65) investigated the radiosensitivity of Lgr5+ crypt base columnar cells (CBCs), a group of ISCs that can reconstitute at least part of the GI tract (66). Hua and colleagues found that radiation exposure caused a dose-dependent decrease in CBCs in the small intestine. In addition, an irreversible loss of CBCs in the small intestine was observed at a radiation dose that caused the GI syndrome (15 Gy). Remarkably, the majority of CBCs were depleted around 1 to 3.5 days, rather than a few hours, after 15 Gy, suggesting that the majority of CBCs died from mitotic death after irradiation. Together, these results reveal a strong association between mitotic catastrophe of CBCs and the onset of the radiation-induced GI syndrome.
Summary
The diverse effect of p53-mediated signaling on radiosensitivity of the GI epithelium reveals the complex biology of the radiation-induced GI syndrome (Table 1). While some crypt epithelial cells are highly sensitive to radiation-induced apoptosis, which is largely dependent on p53 activation, blocking the intrinsic pathway of apoptosis in the GI epithelium does not significantly influence the GI syndrome. In contrast, studies with p53-/- and p21-/- mice demonstrate the significance of the p53/p21-mediated cell cycle arrest pathway in preventing mitotic catastrophe in crypt epithelial cells after irradiation (Figure 1B). Given that multiple types of ISCs may contribute to regeneration of the small intestine after radiation injury (67), future studies using mouse genetics to manipulate p53 expression in specific types of ISCs would provide insight into how p53-mediated apoptosis and cell cycle arrest cooperate to regulate the radiation-induced GI syndrome.
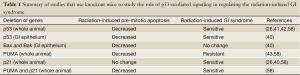
Full table
Role of p53-mediated signaling in response of endothelial cells to radiation
The vascular endothelium is critical to maintain the architecture and function of blood vessels. Damage to endothelial cells significantly contributes to the pathogenesis of acute and late effects of radiation (68,69). For example, animal models show that radiation causes ultrastructural endothelial degeneration and a substantial decrease in microvessel density in the myocardium, which occurs prior to the onset of radiation-induced myocardial injury (70-74). Radiation causes endothelial cell death or dysfunction through a variety of mechanisms including apoptosis (75), senescence (76,77) and mitotic death (77). In vitro studies using endothelial cells from different sources indicate that radiation induces expression of p53 protein and its transcriptional targets, such as the cyclin-dependent kinase inhibitor p21. However, the mechanism through which p53 influences radiation response in endothelial cells is controversial. Some studies indicate that blocking p53-mediated signaling improves survival of endothelial cells in vitro by suppressing apoptosis or senescence (76,78), while others using endothelial cells isolated from p53-/- mice to show that deletion of p53 sensitizes endothelial cells to radiation in vitro (44).
Burdelya et al. evaluated the effect of blocking p53 in tumor stroma, which contains endothelial cells, on tumor response to radiation in vivo. They used mouse tumorigenic packaging cells that produce a retrovirus encoding a dominant-negative mutant p53 to generate xenograft tumors with p53-deficient stroma (44). Tumors with p53-deficient stroma showed markedly prolonged growth delay compared to tumors with p53-wild type stroma. A similar level of growth delay was also observed in tumors that were treated with a p53 inhibitor, PFTα, in combination with radiation. In addition, blocking p53 in tumor stroma resulted in a significant decrease in vessel density in tumors, suggesting that inhibition of p53 sensitizes tumor-associated endothelial cells to radiation in vivo.
To specifically investigate the effect of blocking p53 in endothelial cells on radiation-induced heart disease, we used the Cre-loxP system to delete p53 in endothelial cells using Tie2Cre and VE-Cadherin-Cre mice (45). We observed that after whole-heart irradiation, mice in which both alleles of p53 are deleted in endothelial cells (i.e., Tie2Cre; p53FL/- or VECre; p53FL/- mice) were sensitized to radiation-induced myocardial injury compared to mice that retained one allele of p53 in endothelial cells (i.e., Tie2Cre; p53FL/+ or VECre; p53FL/+ mice). After whole-heart irradiation, both Tie2Cre; p53FL/- and VECre; p53FL/- mice showed a focal decrease in microvessel density in the myocardium, which leads to cardiac ischemia and myocardial necrosis. The progression of myocardial necrosis resulted in systolic dysfunction and heart failure. In addition, in vitro studies using primary endothelial cells showed that after irradiation a higher percentage of p53-deficient endothelial cells displayed early entry into mitosis and contained micronuclei with positive γ-H2AX foci, which result from improper segregation of genomic DNA after radiation. Together, these results demonstrate that p53 protects cardiac endothelial cells from radiation in vivo by preventing the formation of aberrant mitosis or mitotic catastrophe.
Because radiation induces p21 expression in cardiac endothelial cells in a p53-depedent manner, we also studied radiation-induced heart disease in p21-/- mice. Remarkably, after whole heart irradiation p21-/- mice phenocopy the sensitivity of Tie2Cre; p53FL/- and VECre; p53FL/- mice to radiation-induced myocardial injury. Similar to Tie2Cre; p53FL/- and VECre; p53FL/- mice, p21-/- mice developed a reduction in microvessel density, increased vascular permeability and myocardial hypoxia prior to the onset of cardiac dysfunction. These data demonstrate a crucial role of the p53/p21 axis in protecting cardiac endothelial cells from radiation (Figure 1C).
Summary
Results from studies in mice indicate that blocking p53 in vivo through either pharmacological inhibition or genetic deletion dramatically increases radiosensitivity of endothelial cells in tumors and in the heart. These findings suggest that p53 may generally play a pro-survival role in endothelial cell in vivo. Thus, genetically engineered mice with endothelial cell-specific deletion of p53 may be useful tools to mechanistically study the impact of vascular injury on acute and late effects of radiation. Given the diversity of gene expression profiles in human endothelial cells isolated from different tissues (79), further studies are warranted to dissect how p53 functions in endothelial cells to regulate the radiation response of different organs.
Conclusions and perspectives
Radiation activates p53-mediated signaling in a variety of cells; however, the consequence of p53 activation is cell-type dependent. Using genetically engineered mouse models to manipulate the expression of p53 in specific cell types in vivo, several groups have begun to mechanistically dissect the role of p53 in regulating radiation response of different organs in a cell-type specific manner. The findings summarized in this review demonstrate how response of p53 to radiation can vary across different organs or even within the same cell lineage (Figure 1). The complexity by which p53 regulates cellular and tissue response to radiation underscores the importance of understanding mechanisms through which individual cell types respond to radiation. These findings may be critical for developing better strategies to ameliorate normal tissue injury from radiation therapy or radiation disasters.
Acknowledgements
We thank David Van Mater and Katherine Castle for critically reviewing the manuscript.
Funding: This work was supported by NIAID grant R01 AI080488 (D.G.K).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Daohong Zhou and Chuan-Yuan Li) for the series “Stem Cells in Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.09.01). The series “Stem Cells in Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer 2003;3:117-29. [PubMed]
- Gudkov AV, Komarova EA. Pathologies associated with the p53 response. Cold Spring Harb Perspect Biol 2010;2:a001180 [PubMed]
- Lindsay KJ, Coates PJ, Lorimore SA, et al. The genetic basis of tissue responses to ionizing radiation. Br J Radiol 2007;80:S2-6. [PubMed]
- Brady CA, Attardi LD. p53 at a glance. J Cell Sci 2010;123:2527-32. [PubMed]
- Takagi M, Absalon MJ, McLure KG, et al. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell 2005;123:49-63. [PubMed]
- Brooks CL, Gu W. p53 ubiquitination: Mdm2 and beyond. Mol Cell 2006;21:307-15. [PubMed]
- Schlereth K, Charles JP, Bretz AC, et al. Life or death: p53-induced apoptosis requires DNA binding cooperativity. Cell Cycle 2010;9:4068-76. [PubMed]
- Murray-Zmijewski F, Slee EA, Lu X. A complex barcode underlies the heterogeneous response of p53 to stress. Nat Rev Mol Cell Biol 2008;9:702-12. [PubMed]
- Purvis JE, Karhohs KW, Mock C, et al. p53 dynamics control cell fate. Science 2012;336:1440-4. [PubMed]
- Fei P, Bernhard EJ, El-Deiry WS. Tissue-specific induction of p53 targets in vivo. Cancer Res 2002;62:7316-27. [PubMed]
- MacCallum DE, Hupp TR, Midgley CA, et al. The p53 response to ionising radiation in adult and developing murine tissues. Oncogene 1996;13:2575-87. [PubMed]
- Mohrin M, Bourke E, Alexander D, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell 2010;7:174-85. [PubMed]
- Insinga A, Cicalese A, Faretta M, et al. DNA damage in stem cells activates p21, inhibits p53, and induces symmetric self-renewing divisions. Proc Natl Acad Sci U S A 2013;110:3931-6. [PubMed]
- Kirsch DG. Using genetically engineered mice for radiation research. Radiat Res 2011;176:275-9. [PubMed]
- Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol 2002;30:513-28. [PubMed]
- Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys 1995;31:1319-39. [PubMed]
- Williams JP, Brown SL, Georges GE, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res 2010;173:557-78. [PubMed]
- Wang Y, Schulte BA, Zhou D. Hematopoietic stem cell senescence and long-term bone marrow injury. Cell Cycle 2006;5:35-8. [PubMed]
- Wang Y, Schulte BA, LaRue AC, et al. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood 2006;107:358-66. [PubMed]
- Meng A, Wang Y, Van Zant G, et al. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res 2003;63:5414-9. [PubMed]
- Chua HL, Plett PA, Sampson CH, et al. Long-term hematopoietic stem cell damage in a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys 2012;103:356-66. [PubMed]
- Marusyk A, Casas-Selves M, Henry CJ, et al. Irradiation alters selection for oncogenic mutations in hematopoietic progenitors. Cancer Res 2009;69:7262-9. [PubMed]
- Pant V, Quintas-Cardama A, Lozano G. The p53 pathway in hematopoiesis: lessons from mouse models, implications for humans. Blood 2012;120:5118-27. [PubMed]
- Lotem J, Sachs L. Hematopoietic cells from mice deficient in wild-type p53 are more resistant to induction of apoptosis by some agents. Blood 1993;82:1092-6. [PubMed]
- Oren M. Relationship of p53 to the control of apoptotic cell death. Semin Cancer Biol 1994;5:221-7. [PubMed]
- Komarova EA, Kondratov RV, Wang K, et al. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene 2004;23:3265-71. [PubMed]
- Villunger A, Michalak EM, Coultas L, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003;302:1036-8. [PubMed]
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 2001;7:683-94. [PubMed]
- Yu H, Shen H, Yuan Y, et al. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood 2010;115:3472-80. [PubMed]
- Shao L, Sun Y, Zhang Z, et al. Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation. Blood 2010;115:4707-14. [PubMed]
- Harvey M, McArthur MJ, Montgomery CA Jr, et al. Spontaneous and carcinogen-induced tumorigenesis in p53-deficient mice. Nat Genet 1993;5:225-9. [PubMed]
- Jacks T, Remington L, Williams BO, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol 1994;4:1-7. [PubMed]
- Marusyk A, Porter CC, Zaberezhnyy V, et al. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol 2010;8:e1000324 [PubMed]
- Bondar T, Medzhitov R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell 2010;6:309-22. [PubMed]
- Shao L, Li H, Pazhanisamy SK, et al. Reactive oxygen species and hematopoietic stem cell senescence. Int J Hematol 2011;94:24-32. [PubMed]
- Wang Y, Liu L, Pazhanisamy SK, et al. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med 2010;48:348-56. [PubMed]
- Serrano M, Lee H, Chin L, et al. Role of the INK4a locus in tumor suppression and cell mortality. Cell 1996;85:27-37. [PubMed]
- Brown JP, Wei W, Sedivy JM. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science 1997;277:831-4. [PubMed]
- van Os R, Kamminga LM, Ausema A, et al. A Limited role for p21Cip1/Waf1 in maintaining normal hematopoietic stem cell functioning. Stem Cells 2007;25:836-43. [PubMed]
- Kirsch DG, Santiago PM, di Tomaso E, et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science 2010;327:593-6. [PubMed]
- Merritt AJ, Potten CS, Kemp CJ, et al. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res 1994;54:614-7. [PubMed]
- Clarke AR, Gledhill S, Hooper ML, et al. p53 dependence of early apoptotic and proliferative responses within the mouse intestinal epithelium following gamma-irradiation. Oncogene 1994;9:1767-73. [PubMed]
- Qiu W, Carson-Walter EB, Liu H, et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell 2008;2:576-83. [PubMed]
- Burdelya LG, Komarova EA, Hill JE, et al. Inhibition of p53 response in tumor stroma improves efficacy of anticancer treatment by increasing antiangiogenic effects of chemotherapy and radiotherapy in mice. Cancer Res 2006;66:9356-61. [PubMed]
- Lee CL, Moding EJ, Cuneo KC, et al. p53 functions in endothelial cells to prevent radiation-induced myocardial injury in mice. Sci Signal 2012;5:ra52. [PubMed]
- Kemp CJ, Wheldon T, Balmain A. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat Genet 1994;8:66-9. [PubMed]
- Shadad AK, Sullivan FJ, Martin JD, et al. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol 2013;19:185-98. [PubMed]
- Moore JV. The ‘gastrointestinal syndrome’ after chemotherapy: inferences from mouse survival time, and from histologically- and clonogenically-defined cell death in intestinal crypts. Br J Cancer Suppl 1986;7:16-9. [PubMed]
- Somosy Z, Horvath G, Telbisz A, et al. Morphological aspects of ionizing radiation response of small intestine. Micron 2002;33:167-78. [PubMed]
- Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature 1977;269:518-21. [PubMed]
- Booth C, Potten CS. Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest 2000;105:1493-9. [PubMed]
- Merritt AJ, Allen TD, Potten CS, et al. Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene 1997;14:2759-66. [PubMed]
- Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ 2008;15:1153-62. [PubMed]
- Vitale I, Galluzzi L, Castedo M, et al. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol 2011;12:385-92. [PubMed]
- Antignani A, Youle RJ. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr Opin Cell Biol 2006;18:685-9. [PubMed]
- Dewson G, Kluck RM. Mechanisms by which Bak and Bax permeabilise mitochondria during apoptosis. J Cell Sci 2009;122:2801-8. [PubMed]
- Clarke AR. Puma: mauling the intestinal crypt. Cell Stem Cell 2008;2:517-8. [PubMed]
- Leibowitz BJ, Qiu W, Liu H, et al. Uncoupling p53 functions in radiation-induced intestinal damage via PUMA and p21. Mol Cancer Res 2011;9:616-25. [PubMed]
- Bunz F, Dutriaux A, Lengauer C, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 1998;282:1497-501. [PubMed]
- Brugarolas J, Chandrasekaran C, Gordon JI, et al. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 1995;377:552-7. [PubMed]
- García-Cao I, Garcia-Cao M, Martin-Caballero J, et al. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J 2002;21:6225-35. [PubMed]
- Sullivan JM, Jeffords LB, Lee CL, et al. p21 protects “Super p53” mice from the radiation-induced gastrointestinal syndrome. Radiat Res 2012;177:307-10. [PubMed]
- Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell 2012;11:452-60. [PubMed]
- Hendry JH, Potten CS. Intestinal cell radiosensitivity: a comparison for cell death assayed by apoptosis or by a loss of clonogenicity. Int J Radiat Biol Relat Stud Phys Chem Med 1982;42:621-8. [PubMed]
- Hua G, Thin TH, Feldman R, et al. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology 2012;143:1266-76. [PubMed]
- Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003-7. [PubMed]
- Yan KS, Chia LA, Li X, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A 2012;109:466-71. [PubMed]
- Wang J, Boerma M, Fu Q, et al. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol 2007;13:3047-55. [PubMed]
- Stewart FA, Hoving S, Russell NS. Vascular damage as an underlying mechanism of cardiac and cerebral toxicity in irradiated cancer patients. Radiat Res 2010;174:865-9. [PubMed]
- Fajardo LF, Stewart JR. Pathogenesis of radiation-induced myocardial fibrosis. Lab Invest 1973;29:244-57. [PubMed]
- Fajardo LF, Stewart JR. Experimental radiation-induced heart disease. I. Light microscopic studies. Am J Pathol 1970;59:299-316. [PubMed]
- Lauk S, Kiszel Z, Buschmann J, et al. Radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys 1985;11:801-8. [PubMed]
- Yeung TK, Lauk S, Simmonds RH, et al. Morphological and functional changes in the rat heart after X irradiation: strain differences. Radiat Res 1989;119:489-99. [PubMed]
- Seemann I, Gabriels K, Visser NL, et al. Irradiation induced modest changes in murine cardiac function despite progressive structural damage to the myocardium and microvasculature. Radiother Oncol 2012;103:143-50. [PubMed]
- Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 2001;293:293-7. [PubMed]
- Lee MO, Song SH, Jung S, et al. Effect of ionizing radiation induced damage of endothelial progenitor cells in vascular regeneration. Arterioscler Thromb Vasc Biol 2012;32:343-52. [PubMed]
- Mendonca MS, Chin-Sinex H, Dhaemers R, et al. Differential mechanisms of x-ray-induced cell death in human endothelial progenitor cells isolated from cord blood and adults. Radiat Res 2011;176:208-16. [PubMed]
- Nübel T, Damrot J, Roos WP, et al. Lovastatin protects human endothelial cells from killing by ionizing radiation without impairing induction and repair of DNA double-strand breaks. Clin Cancer Res 2006;12:933-9. [PubMed]
- Chi JT, Chang HY, Haraldsen G, et al. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci U S A 2003;100:10623-8. [PubMed]


