
Exosomes in semen: opportunities as a new tool in prostate cancer diagnosis
Introduction
There is a growing interest in the use of a minimally invasive liquid biopsy to identify biomarkers in several cancers, including urologic malignancies (1). Liquid biopsy and in particular seminal exosomes (SE) recovered by seminal fluid (SF) could represent an important innovation in the field of precision medicine. Precision medicine can be applied in screening, diagnosis, prognosis, prediction of treatment response and resistance, early detection of metastasis and biologic cancer stratification (2).
Prostate cancer (PCa) is the most common cancer in men; it is normally diagnosed through the test of serum prostate-specific antigen (PSA) and rectal examination. Since PCa is a highly heterogeneous cancer, there are currently many conflicting opinions about PSA-based diagnosis (3).
There is an urgent need for a non-invasive test to select non-metastatic from metastatic PCa. Since 40% of prostatic material is SF, using this fluid as source of biomarkers might have some advantages in comparison with blood and urine. SF, highly enriched in prostatic constituents compared to other body fluids (4,5), is composed of gland secretions in the male urogenital tract. Other two SF components are the seminal vesicles and testes. SF is released from smooth muscle contraction following an expulsion into the urethra. Moreover, prostate cells and their secretions are released naturally into SF by both normal and malignant glands. SF contains cell-free material released by PCa cells, that could permit to detect PCa easier than biopsy (6). For these reasons, SF could be a good source of biomarkers for early detection, thus the use of liquid biopsy for prostate analyses could improve diagnosis of PCa with relevant clinical advantages.
PCa
PCa is the most frequent cancer diagnosed in men (7), but for prostate tumor heterogeneity, prostate biopsies analysis underestimates the grade of pathology. In order to have more information about the prostate status, blood or urine biomarkers could be useful. New efficient biomarkers could make active PC surveillance less invasive, reduce costs and eliminate the complications related to biopsy samples.
Currently, first line blood-derived molecular biomarker to diagnose of PCa is PSA, which is a protease, produced by prostate epithelial cells to liquefy the semen coagulum. PSA is mainly deposited into prostate ducts, but small amounts of it can also be detected in the healthy individual’s blood (8). Transformed PCa cells increase the release of PSA into blood. Nowadays, there are many limitations linked to PSA’s usage in clinical application. PSA levels indicate not only small, localized, and low-grade malignant tumors, but also benign prostatic hyperplasia and prostatitis or urinary tract infection. Besides these are highly variable between healthy individuals. PSA lacks enough specificity to distinguish efficiently differences between benign prostate disease and aggressive PCa (9,10). Another molecular biomarker tested in PCa is PCa antigen 3 (PCA3). PCA3 is a PCa-specific antigen, overexpressed in more than 90% of PCa that can also be detected in urine (11). PCA3 is a noncoding RNA (ncRNA) that may have higher specificity for the prediction of PCa in comparison with PSA, but the sensitivity is relatively low (12).
Furthermore, urine-based tests of the fusion gene transcript can be used as PCa markers; chromosomal re-arrangements that lead to gene fusion are a common feature of carcinomas. Frequent gene fusions in PCa are androgen-regulated gene transmembrane protease serine 2 (TMPRSS2) and two ETS transcription factors, ETV1 and the v-ets erythroblastosis virus E26 oncogene homolog (ERG) (13). Unfortunately, the analyses of the fusion gene transcript TMPRSS2 and ERG, show high specificity and low sensitivity.
The discovery of new PCa markers in SF, prostate-specific blood and urine extracellular vesicles (EVs) are considered as non-invasive potential source of biomarkers for PCa.
SE
SF contains high concentration (about 1012 or more purified particles per ejaculate) of subcellular lipid-bound microparticles (14) derived from male genital tract such as epididymal ducts, vesicular glands, and bulbourethral glands (15) and vesicles derived from prostate gland’s epithelial cells traditionally termed “prostasomes” (16) (Figure 1). Overall, this vesicle population is usually denominated SE. SE are similar to exosomes, released by other cells, for their morphological features (cup shape and diameter size) and canonical biochemical exosomal markers, such as HSP70 and CD63 (14). SE have a role in immune-suppression (17) and affects the complement system (18). It was reported that exposure of the female reproductive tract to semen results in immune-modulatory events which influence the outcome of HIV-1 replication within the genital mucosa (19,20). HIV-1 and human papilloma virus (HPV) is transmitted primarily through sexual contact and semen is the primary vector (21). HPV is the principal factor involved in the development of cervical carcinoma. In this common cancer, semen is responsible of HPV delivery and caused the continuous exposure of the cervix to immune-suppressive agents. The correlation between SE, immunity and HIV-1 transmission have been demonstrated (22). SE may either enhance or block replication of sexually transmitted pathogens, such as HIV-1 (19,20). Madison et al. showed that semen exosomes purified from healthy human donors alter HIV-1 replication, through alteration in intravirion reverse transcriptase (RT) activity and protein composition, drastically decreasing HIV infectivity (23). HIV-1 RT is a heterodimer composed from p66 and p51 kDa subunits. The association of these polypeptides is necessary for RT enzymatic activity, because monomeric subunit lacks polymerase activity (24,25). It was demonstrated that p66 RT subunit was absent from virions generated in the presence of SE. It was also showed that SE contain Apobec3, a potent antiretroviral, mediating inhibition of retroviruses (26).
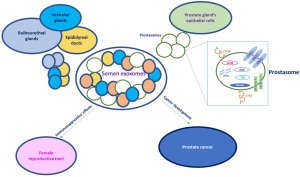
Moreover, neutrophils and monocytes have the capacity to undergo phagocytosis and generate oxidative burst responses in the female reproductive tract during the insemination. Large numbers of natural killer (NK) cells have been found in the female reproductive tract; they create a hostile environment for spermatozoa. Flow cytometric analysis revealed that prostasomes expressed high levels of CD48, ligand for the activating CD244 receptor, expressed on NK cells and mediates non-major histocompatibility complex restricted killing. Tarazona et al., reported that the interaction between NK-cells and target cells, via this receptor, modulate NK-cell cytolytic activity. Interactions between NK cells and purified prostasomes resulted in a decrease of CD244 expression. Moreover, the decreased NK cell activity cultured in the presence of prostasomes, suggests that these vesicles may immune-modulate the local environment within the female reproductive tract, preventing the immune-mediated sperm destruction and prolonging the sperms’ survival rate (27). It is also suggested that the immune-suppressive activity of the human SF components protect inseminated sperms in female reproductive tract (28); stimulating pre-cancerous cells to progress to full carcinoma (29).
SE cargo includes cytokines, growth factors and membrane proteins, as well as messenger RNAs and microRNAs (miRs) that affect the recipient cells (30). Vojtech et al. suggested that SEs, carry non-coding RNA molecules that regulate biological functions through degradation or inhibition of specific mRNA targets (14).
The immunosuppressive effects of SE are partially mediated by the activities of these regulatory RNAs. SE delivered in high concentration in a limited anatomical area of the female genital tract could increase an immune-modulation mediated by exosomes (14).
Few studies have shown that immune-related mRNAs are targeted by miRs carried by semen exosomes, altering the normal immune response to pathogens. It is demonstrated that miR-148a, contained in SE, is able to target calcium/calmodulin-dependent protein kinase II (CaMKII) in dendritic cells. This process lead to the reduction of MHC II expression and secretion of cytokines, and inhibition of dendritic cells-mediated T cell expansion (31). Let-7 family members are also abundant in semen exosomes; these miRs control the IL-10 and IL-13 expression, in macrophages following pathogenic stimulus, as well as IL-13 in T cells (32,33).
Role of prostasomes in PCa
Among the vesicles isolated from semen, only prostasomes (34) is truly derived from the prostatic cells. These vesicles are spherical nanoparticles with a diameter of 50 to 500 nm, released by prostatic epithelial cells in the extracellular media. These vesicles, described for the first time in 1982, promote motility of sperm cells (35); regulate sperm cell capacitation, acrosome reaction, and immune suppression within the female reproductive tract (16,36). Prostasomes molecular composition could reflect their capacity to influence PCa growth and metastasis. Proteomic profile of prostasome isolated from SF identify prostate-specific membrane proteins (like TMPRSS2), prostate-specific transglutaminase, and prostate stem cell antigen (PSCA), confirmed the prostatic origin of these vesicles (16,37,38). The first efforts to identify the prostasomes in a PCa patient’s blood was the detection of anti-prostasome antibodies (39-41). In healthy individuals, excretory ducts form a closed compartment with the basement membrane surrounding the prostate epithelial cells, in which prostasomes from the immune system hide. The loss of cell polarity in PCa (42) allows the release of prostasomes into microenvironment and circulation (43,44). This process induces the adaptive immune system to produce prostasome-directed autoantibodies, which can be isolated by PCa patients’ blood (39,41). However, antibodies against prostasome cannot be quantified and their correlation with PCa grade or metastasis it is not possible (40,45), showing the low efficacy of prostasome-specific antibodies as reliable prognostic markers for PCa (46).
Unfortunately, not only neoplastic prostate cells but also normal prostate acinar secretory cells have the capacity to release and export prostasomes to the extracellular space (43,47). Prostasomes can assist the fertilization of the sperm cells (48), induce the transition of normal cell to neoplastic one and help the poorly differentiated cancer cells to survive (29). Prostasomes protein kinases and phosphatases control cell proliferation, differentiation and signal transduction pathways (49), and regulating cell malignant transformation. Literature data showed that protein kinase activities are up regulated in prostasomes released from malignant cells (50). Other proteins and enzymes related to malignant transformation and proliferation are expressed on the prostasome surface such as protectin (51) or tissue factor (52) and matrix metalloproteinase (53). Moreover, prostasomes derived from PCa metastases showed higher levels of annexins A1, A3 and especially annexin A5, compared to prostasomes derived from non-metastatic PCa (54). In mammals, the annexin family consists in 12 calcium ion-dependent phospholipid-binding proteins that are implicated in cell differentiation, immunomodulation and migration. Annexin A1 was first identified as a mediator of the anti-inflammatory activity of glucocorticoids (55). Annexin A3 promotes tumour growth and angiogenesis inducing vascular endothelial growth factor (VEGF) expression (56). Annexin A5 is associated with the inhibition of phospholipase A2, a membrane-bound prostasome enzyme of human SF (57). Furthermore, dimethylarginine dimethylaminohydrolase 1 (DDAH-1) is observed in prostasomes derived from PCa metastasis (54). DDAH-1 induces nitric oxide (NO) synthesis (58) that is a crucial regulator of angiogenesis (59). These reports indicate that prostasomes have a key role in several steps of PCa progression.
Clinical application of SE
Among the SE, prostasomes contain PCa-specific molecular fingerprints that could represent the status of their originating cells. The presence of EVs from many other sources makes the detection of prostasomes in blood even more difficult. Nevertheless, it was also reported that tumor suppressor PTEN was approximately 10-fold higher in EVs isolated from PCa patients compared with normal subjects (60). Considering the interest in the application of exosomes as non-invasive cancer biomarkers, recently it was developed ExoDx™ Prostate (IntelliScore), a FDA-approved non-invasive urine test to analyse the expression of three exosomal RNAs associated with high-grade PCa. This test is used with PSA to distinguish high grade (Gleason score ≥GS7) from low grade cancers (61). Despite tests on blood samples already developed, the advantage of collecting EVs from urine is the enrichment in prostasomes rather than other constituents. The presence of prostatic fluid into urine and prostasomes in EVs fractions isolated from urine was confirmed by detection of prostate-specific proteins like prostate-specific membrane antigen (PSMA), prostatic acid phosphatase, and prostate transglutaminase (62-65). In a recent study, protein compositions of EVs, isolated from preoperative urine samples of PCa patients was compared to the samples of healthy individuals. Mass spectrometry revealed that among the differentially expressed proteins, the transmembrane protein TM256 displayed the highest sensitivity (66). TM256 could be used as biomarkers to select PCa patients. Conversely, the limit to use urine-derived EVs is the variability of their concentration; they are strongly influenced by external factors such as prostate massage, the timing, frequency, and volume of urination.
SE can be used, in the liquid biopsies scenario, in order to provide a new tool for early diagnosis of tumors of the urogenital tract. Furthermore, these vesicles could be used to monitor the efficacy of the therapy or as shuttle of therapeutical compounds.
Regarding these last aspects, it is important considered long-term stability of SE components. A recent study showed that prolonged semen freezing has no significant effect on the recovery of semen exosomes, but the levels of specific SE cargoes may be altered, as indicated by decreased AChE activity. AChE is a plasma membrane protein incorporated into exosomes during exosome biogenesis. The enzymatic activity of AChE as commonly used as exosome marker (67). AChE inhibition downregulates HIV1-induced T cell activation and T cell proliferation in chronically infected HIV-1 patients (68). Thus, decreased AChE activity in semen’s exosomes after prolonged frozen storage of the semen may have important biological effects in clinical application of these exosomes. Nevertheless, prolonged freezing of semen has no effect on exosomal CD63 and CD9 at protein and mRNA levels. CD63, CD9 and other tetraspanin proteins play multiple important roles in HIV-1 infection (69,70). CD63 are incorporated into released HIV-1 particles. Although the role of CD9, in HIV infection, has not been extensively studied. It has been demonstrated that overexpression of CD9 can also reduce HIV-1 infectivity (70,71).
Conclusions
In the era of precision medicine, the goal of new therapeutic approaches is to eliminate the “one size fits all” model of patient management. Liquid biopsy is a promising non-invasive tool for molecular profiling, to allow the evaluation of circulating molecules, in several biological fluids and in particular in EVs for biomarker discovery (1). Overall, the reports discussed in this review indicate that SE could represent a new tool for diagnosis, prognosis and monitoring of urogenital cancers. Other studies are necessary to improve the knowledge about SE to highlight the role of SE as biomarkers in urogenital cancers.
Acknowledgments
E Durendez has a predoctoral fellowship by Asociación Española Contra el Cáncer, Valencia (AECC Valencia).
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Edward R. Sauter) for the series “Body Fluid Exosomes and Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.10.25). The series “Body Fluid Exosomes and Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Di Meo A, Bartlett J, Cheng Y, et al. Liquid biopsy: a step forward towards precision medicine in urologic malignancies. Mol Cancer 2017;16:80. [Crossref] [PubMed]
- Pasic MD, Samaan S, Yousef GM. Genomic medicine: new frontiers and new challenges. Clin Chem 2013;59:158-67. [Crossref] [PubMed]
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320-8. [Crossref] [PubMed]
- Gardiner RA, Burger M, Clements JA, et al. Realizing the potential of ejaculate/seminal fluid in detecting and predicting natural history. Methods Mol Med 2003;81:199-217. [PubMed]
- Roberts MJ, Schirra HJ, Lavin MF, et al. Metabolomics: a novel approach to early and noninvasive prostate cancer detection. Korean J Urol 2011;52:79-89. [Crossref] [PubMed]
- Hayes GM, Simko J, Holochwost D, et al. Regional cell proliferation in microdissected human prostate specimens after heavy water labeling in vivo: correlation with prostate epithelial cells isolated from seminal fluid. Clin. Cancer Res 2012;18:3250-60. [Crossref] [PubMed]
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence mortality in Europe in 2008. Eur J Cancer 2010;46:765-81. [Crossref] [PubMed]
- Saini S. PSA and beyond: alternative prostate cancer biomarkers. Cell Oncol (Dordr) 2016;39:97-106. [Crossref] [PubMed]
- Huang YQ, Sun T, Zhong WD, et al. Clinical performance of serum [-2]proPSA derivatives, %p2PSA and PHI, in the detection and management of prostate cancer. Am J Clin Exp Urol 2014;2:343-50. [PubMed]
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med 2004;350:2239-46. [Crossref] [PubMed]
- Salagierski M, Schalken JA. Molecular diagnosis of prostate cancer: PCA3 and TMPRSS2:ERG gene fusion. J Urol 2012;187:795-801. [Crossref] [PubMed]
- Stephan C, Ralla B, Jung K. Prostate-specific antigen and other serum and urine markers in prostate cancer. Biochim Biophys Acta 2014;1846:99-112. [PubMed]
- Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005;310:644-8. [Crossref] [PubMed]
- Vojtech L, Woo S, Hughes S, et al. Exosomes in human semencarry a distinctiverepertoire of small non-codingRNAs with potential regulatory functions. Nucleic Acids Res 2014;42:7290-304. [Crossref] [PubMed]
- Renneberg H, Konrad L, Dammshäuser I, et al. Immunohistochemistry of prostasomes from human semen. Prostate 1997;30:98-106. [Crossref] [PubMed]
- Aalberts M, Stout TA, Stoorvogel W. Prostasomes: extracellular vesicles from the prostate. Reproduction 2013;147:R1-14. [Crossref] [PubMed]
- Skibinski G, Kelly RW, Harkiss D, et al. Immunosuppression by human seminal plasma–extracellular organelles (prostasomes) modulate activity of phagocytic cells. Am J Reprod Immunol 1992;28:97-103. [Crossref] [PubMed]
- Babiker AA, Ronquist G, Nilsson UR, et al. Transfer of prostasomal CD59 to CD59-deficient red blood cells results in protection against complement-mediated hemolysis. Am J Reprod Immunol 2002;47:183-92. [Crossref] [PubMed]
- Martellini JA, Cole AL, Svoboda P, et al. HIV-1 enhancing effect of prostatic acid phosphatase peptides is reduced in human seminal plasma. PLoS One 2011;6:e16285 [Crossref] [PubMed]
- Roan NR, Liu H, Usmani SM, et al. Liquefaction of semen generates and later degrades a conserved semenogelin peptide that enhances HIV infection. J Virol 2014;88:7221-34. [Crossref] [PubMed]
- Royce RA, Sena A, Cates W Jr, et al. Sexual transmission of HIV. N Engl J Med 1997;336:1072-8. [Crossref] [PubMed]
- Madison MN, Jones PH, Okeoma CM. Exosomes in human semen restrict HIV-1 transmission by vaginal cells and block intravaginal replication of LP-BM5 murine AIDS virus complex. Virology 2015;482:189-201. [Crossref] [PubMed]
- Madison MN, Roller RJ, Okeoma CM. Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology 2014;19;11:102.
- Tachedjian G, Radzio J, Sluis-Cremer N. Relationship between enzyme activity and dimeric structure of recombinant HIV-1 reverse transcriptase. Proteins 2005;60:5-13. [Crossref] [PubMed]
- Tachedjian G, Aronson HE, de los Santos M, et al. Role of residues in the tryptophan repeat motif for HIV-1 reverse transcriptase dimerization. J Mol Biol 2003;326:381-96. [Crossref] [PubMed]
- Okeoma CM, Low A, Bailis W, et al. Induction of APOBEC3 in vivo causes increased restriction of retrovirus infection. J Virol 2009;83:3486-95. [Crossref] [PubMed]
- Tarazona R, Delgado E, Guarnizo MC, et al. Human prostasomes express CD48 and interfere with NK cell function. Immunobiology 2011;216:41-6. [Crossref] [PubMed]
- Binks S, Pockley AG. Modulation of leukocyte phagocytic and oxidative burst responses by human seminal plasma. Immunol Invest 1999;28:353-64. [Crossref] [PubMed]
- Ronquist G, Nilsson BO. The Janus-faced nature of prostasomes: their pluripotency favours the normal reproductive process and malignant prostate growth. Prostate Cancer Prostatic Dis 2004;7:21-31. [Crossref] [PubMed]
- Turchinovich A, Samatov TR, Tonevitsky AG, et al. Circulating miRNAs: cell-cell communication function? Front Genet 2013;4:119. [Crossref] [PubMed]
- Liu X, Zhan Z, Xu L, et al. MicroRNA-148/152 impair innate response and antigen presentation of TLR-triggered dendritic cells by targeting CaMKIIalpha. J. Immunol 2010;185:7244-51. [Crossref] [PubMed]
- Kumar M, Ahmad T, Sharma A, et al. Let-7 microRNA-mediated regulation of IL-13 and allergic airway inflammation. J Allergy Clin Immunol 2011;128:1077-85.e1-10.
- Schulte LN, Eulalio A, Mollenkopf HJ, et al. Analysis of the host microRNA response to Salmonella uncovers the control of major cytokines by the let-7 family. EMBO J 2011;30:1977-89. [Crossref] [PubMed]
- Zijlstra C, Stoorvogel W. Prostasomes as a source of diagnostic biomarkers for prostate cancer. J Clin Invest 2016;126:1144-51. [Crossref] [PubMed]
- Stegmayr B, Ronquist G. Promotive effect on human sperm progressive motility by prostasomes. Urol Res 1982;10:253-7. [Crossref] [PubMed]
- Ronquist G. Prostasomes are mediators of intercellular communication: from basic research to clinical implications. J Intern Med 2012;271:400-13. [Crossref] [PubMed]
- Utleg AG, Yi EC, Xie T, et al. Proteomic analysis of human prostasomes. Prostate 2003;56:150-61. [Crossref] [PubMed]
- Poliakov A, Spilman M, Dokland T, et al. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate 2009;69:159-67. [Crossref] [PubMed]
- Nilsson BO, Carlsson L, Larsson A, et al. Autoantibodies to prostasomes as new markers for prostate cancer. Ups J Med Sci 2001;106:43-9. [Crossref] [PubMed]
- Minelli A, Ronquist G, Carlsson L, et al. Antiprostasome antibody titres in benign and malignant prostate disease. Anticancer Res 2005;25:4399. [PubMed]
- Ronquist KG, Carlsson L, Ronquist G, et al. Prostasome-derived proteins capable of eliciting an immune response in prostate cancer patients. Int J Cancer 2006;119:847-53. [Crossref] [PubMed]
- Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev 2000;14:2410-34. [Crossref] [PubMed]
- Nilsson BO, Egevad L, Jin M, et al. Distribution of prostasomes in neoplastic epithelial prostate cells. Prostate 1999;39:36-40. [Crossref] [PubMed]
- Tavoosidana G, Ronquist G, Darmanis S, et al. Multiple recognition assay reveals prostasomes as promising plasma biomarkers for prostate cancer. Proc Natl Acad Sci U S A 2011;108:8809-14. [Crossref] [PubMed]
- Larsson A, Ronquist G, Wülfing C, et al. Antiprostasome antibodies: possible serum markers for prostate cancer metastasizing liability. Urol Oncol 2006;24:195-200. [Crossref] [PubMed]
- Stewart AB, Delves GH, Birch BR, et al. Antiprostasome antibodies are not an appropriate prognostic marker for prostate cancer. Scand J Urol Nephrol 2009;43:104-8. [Crossref] [PubMed]
- Floryk D, Tollaksen SL, Giometti CS, et al. Differentiation of human prostate cancer PC-3 cells induced by inhibitors of inosine 5’-monophosphate dehydrogenase. Cancer Res 2004;64:9049-56. [Crossref] [PubMed]
- Arienti G, Carlini E, Nicolucci A, et al. The motility of human spermatozoa as influenced by prostasomes at various pH levels. Biol Cell 1999;91:51-4. [Crossref] [PubMed]
- Wilson MJ. Protein kinases and protein phosphorylation in prostasomes. In: Prostasomes. Ronquist G, Nilsson BO. editors. Stockholm: Portland Press Ltd. London, 2001:145-53.
- Babiker AA, Ronquist G, Nilsson B, et al. Overexpression of ecto-protein kinases in prostasomes of metastatic cell origin. Prostate 2006;66:675-86. [Crossref] [PubMed]
- Babiker AA, Nilsson B, Ronquist G, et al. Transfer of functional prostasomal CD59 of metastatic prostatic cancer cell origin protects cells against complement attack. Prostate 2005;62:105-14. [Crossref] [PubMed]
- Babiker AA, Hamad OA, Sanchez J, et al. Prothrombotic effect of prostasomes of metastatic cell and seminal origin. Prostate 2007;67:378-88. [Crossref] [PubMed]
- Bellezza I, Aisa MC, Palazzo R, et al. Extracellular matrix degrading enzymes at the prostasome surface. Prostate Cancer Prostatic Dis 2005;8:344-8. [Crossref] [PubMed]
- Ronquist KG, Ronquist G, Larsson A, et al. Proteomic analysis of prostate cancer metastasis-derived prostasomes. Anticancer Res 2010;30:285-90. [PubMed]
- Moss SE, Morgan RO. The annexins. Genome Biol 2004;5:219. [Crossref] [PubMed]
- Park JE, Lee DH, Lee JA, et al. Annexin A3 is a potential angiogenic mediator. Biochem Biophys Res Commun 2005;337:1283-7. [Crossref] [PubMed]
- Lindahl M, Tagesson C, Ronquist G. Phospholipase A2 activity in prostasomes from human seminal plasma. Urol Int 1987;42:385-9. [Crossref] [PubMed]
- Knipp M, Charnock JM, Garner CD, et al. Structural and functional characterization of the Zn(II) site in dimethylargininase-1 (DDAH-1) from bovine brain. Zn(II) release activates DDAH-1. J Biol Chem 2001;276:40449-56. [Crossref] [PubMed]
- Choudhari SK, Chaudhary M, Bagde S, et al. Nitric oxide and cancer: a review. World J Surg Oncol 2013;11:118. [Crossref] [PubMed]
- Gabriel K, Ingram A, Austin R, et al. Regulation of the tumor suppressor PTEN through exosomes: a diagnostic potential for prostate cancer. PLoS One 2013;8:e70047 [Crossref] [PubMed]
- McKiernan J, Donovan MJ, O'Neill V, et al. A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA Oncol 2016;2:882-9. [Crossref] [PubMed]
- Nilsson J, Skog J, Nordstrand A, et al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer 2009;100:1603-7. [Crossref] [PubMed]
- Huang X, Yuan T, Liang M, et al. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol 2015;67:33-41. [Crossref] [PubMed]
- Kim Y, Ignatchenko V, Yao CQ, et al. Identification of differentially expressed proteins in direct expressed prostatic secretions of men with organ-confined versus extracapsular prostate cancer. Mol Cell Proteomics 2012;11:1870-84. [Crossref] [PubMed]
- Mitchell PJ, Welton J, Staffurth J, et al. Can urinary exosomes act as treatment response markers in prostate cancer? J Transl Med 2009;7:4. [Crossref] [PubMed]
- Øverbye A, Skotland T, Koehler CJ, et al. Identification of prostate cancer biomarkers in urinary exosomes. Oncotarget 2015;6:30357-76. [Crossref] [PubMed]
- de Gassart A, Geminard C, Fevrier B, et al. Lipid raft-associated protein sorting in exosomes. Blood 2003;102:4336-44. [Crossref] [PubMed]
- Valdés-Ferrer SI, Crispín JC, Belaunzarán PF, et al. Acetylcholine-esterase inhibitor pyridostigmine decreases T cell overactivation in patients infected by HIV. AIDS Res Hum Retroviruses 2009;25:749-55. [Crossref] [PubMed]
- Ho SH, Martin F, Higginbottom A, et al. Recombinant extracellular domains of tetraspanin proteins are potent inhibitors of the infection of macrophages by human immunodeficiency virus type 1. J Virol 2006;80:6487-96. [Crossref] [PubMed]
- Krementsov DN, Weng J, Lambele M, et al. Tetraspanins regulate cell-to-cell transmission of HIV-1. Retrovirology 2009;6:64. [Crossref] [PubMed]
- Sato K, Aoki J, Misawa N, et al. Modulation of human immunodeficiency virus type 1 infectivity through incorporation of tetraspanin proteins. J Virol 2008;82:1021-33. [Crossref] [PubMed]


