
Trans-abdominal ultrasound features of the newly named intraductal papillary neoplasm of the bile duct
Introduction
Intraductal papillary mucinous neoplasm (IPMN) of the bile duct (IPMN-B) is a newly recognized disease, added to the World Health Organization (WHO) disease classification in 2010 (1). IPMN-B is considered a type of IPMN specific to the bile duct.
IPMN-B derives from an IPMN of the pancreas (IPMN-P) first reported by Ohashi et al. (2). in 1982 and named by the WHO in 1996. IPMN-P was described as papillary epithelial tumors originating from the pancreatic duct with macroscopically visible mucin hyper-secretion. IPMN-B is similar to IPMN-P in clinical and pathologic behavior (3,4), but IPMN-B originates from the epithelial cells of the bile duct and has the potential to progress into malignant lesions. IPMN-B constitutes precancerous lesions of bile duct carcinoma. Because trans-abdominal ultrasound is the first choice for characterizing bile duct disease, describing the ultrasound features of this new disease is important to aid early diagnosis, surgical resection, and improve prognosis.
To our knowledge, the only previous reports of IPMN-B have been retrospective analyses with a small sample, or case reports. Tsuyuguchi et al. (5) conducted a retrospective analysis concerning the clinical features and endoscopic diagnostic strategy for eight cases. Lim et al. (6) analyzed six patients with IPMN-B that manifested only as dilation of the segmental or lobar hepatic duct. Guo et al. (7) reported a case of IPMN-B in the left hepatic lobe diagnosed via endoscopic ultrasonography. There have been no preoperative diagnoses of IPMN-B, due to its relative rarity and the lack of specific known clinical and imaging manifestations. Therefore, studies with larger populations are needed to discern the diagnostic clinical features of IPMN-B. As the preferred imaging alternative for biliary disease, it is important to determine the imaging characteristics of IPMN-B gained from conventional trans-abdominal ultrasound.
To determine possible diagnostic features on trans-abdominal ultrasound, we retrospectively categorized types of IPMN-B based on trans-abdominal ultrasound findings in a relatively large sample of 20 patients, and compared them for clinical and pathological features.
Methods
Patients
We collected the records of all cases of intrahepatic cystic lesions with papillary tumor from 2002 to 2016 that were confirmed by the Pathology Department of Peking Union Medical College Hospital, the First Affiliated Hospital of PLA General Hospital, and Tsinghua Chang Gung Hospital. The slides were reviewed again by two senior experienced hepatic-biliary pathologists at Peking Union Medical College Hospital. By consensus, 28 cases were diagnosed as IPMN-B, among which 20 had complete TAU data and were selected for this study.
All procedures performed in studies were in accordance with the ethical standards of The Institutional Review Board (IRB) of Peking Union Medical College Hospital (PUMCH).
Trans-abdominal ultrasound examinations
After fasting for 8 hours, each patient was subjected to conventional TAU using a color Doppler ultrasound device (Philips iU22 or GE Logiq 9) with a 2.0–5.0 MHz convex probe. The obtained ultrasound results were retrospectively reviewed by two doctors with 20 years of experience in abdominal ultrasound. All 20 patients were evaluated based on the location and structure of the mass, the relationships between the mass and the bile duct, the dilation of the bile duct.
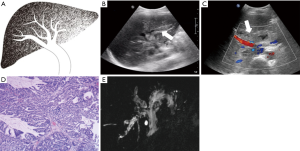
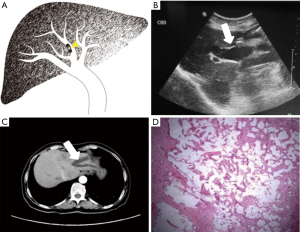
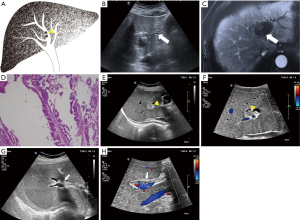
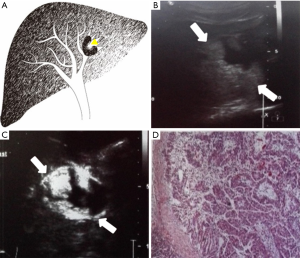
Each case was classified as one of four types (I–IV) of IPMN-B, based on the ultrasound imaging features and the previous experience of Lim et al. (8) regarding the relationships between the dilated bile duct and the mass (Table 1; Figures 1-4).

Full table
Also collected for each patient were the clinical features, pathological results, and serum concentrations of the tumor markers carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA), and alpha-fetoprotein (AFP).
Statistical analysis
The serum concentrations of each of the tumor markers and the pathological results of the different IPMN-B types were evaluated by Fisher’s exact test. One-way analysis of variance was used to compare the sizes of the common bile duct. P<0.05 was considered a statistically significant difference. SPSS 16.0 was used for all data analyses.
Results
Clinical and pathological features
The 20 patients with IPMN-B were 9 men and 11 women, and the number of each gender was comparable (Table 2). The average age was 60±12 years (range, 26–73 years).
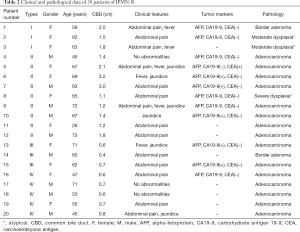
Full table
The average time from first self-conscious symptom to surgery was 46 months (range, 0.5–240 months). The most common clinical symptom observed was abdominal pain (14 patients), which was accompanied by fever or jaundice in five patients. Of the 6 remaining patients without abdominal pain, 3 had fever or jaundice and 3 had no significant symptoms. Of the 20 patients in this study, 12 had records for serum concentrations of tumor markers. Of those 12, 6 showed elevated results: 5 with elevated CA19-9, and 1 with elevated CEA. These 6 cases were all malignant. The remaining 6 patients with serum marker results included 3 benign and 3 malignant cases, each with normal levels (P=0.046).
All patients underwent hepatectomy, that is, 18 underwent left lobectomy, and one each were given right lobectomy or caudate lobectomy. Pathological results showed that 15, 3, and 2 cases were adenocarcinoma, atypical hyperplasia, and border adenoma, respectively. Due to lack of knowledge of IPMN-B at the time of presentation, 12 of the 20 patients were originally given a diagnosis of mucinous cystic neoplasm (MCN), 7 were considered IPMN-B, and one as cholangiocarcinoma.
Ultrasound findings
The conventional trans-abdominal ultrasound findings showed no hepatic mass in three patients (type I; Table 3). Of the 17 patients with a hepatic mass (types II–IV), there were 15 in the left lobe, and one each in the right lobe and caudate lobe. All benign atypical hyperplasia cases, and 50% of the border adenoma cases, belonged to types I or II. Types III and IV more aggressively progressed to adenocarcinoma.

Full table
The diameters of the common bile duct of the I–IV ultrasound types were 2.27±1.08, 1.82±0.79, 0.57±0.15, and 0.68±0.08 cm, respectively, with types I and II significantly larger than types III and IV. The distribution of malignant cases among the four ultrasound types differed significantly (P=0.008, Table 4).

Full table
The ultrasound images revealed a dilated biliary duct in 15 cases: 3, 9, and 3 cases of types I, II, and III, respectively. In the type I group, the three patients showed only diffuse dilation of both the intrahepatic and extrahepatic bile duct, without any visible tumor. In the type II group, the 9 cases were both intrahepatic and extrahepatic, without obstruction caused by tumors located in the left lobe. In the type III group, the 3 cases were all intrahepatic, with complete or partial obstruction caused by tumors of the left lobe.
The 5 cases of type IV comprised 3, 1, and 1 tumor in the left lobe, right lobe, and caudate lobe, respectively. These cases showed only aneurysmal masses and intra-cystic papillary neoplasm, without any visible proximal or distal bile duct dilation.
Discussion
This retrospective study investigated trans-abdominal ultrasound findings for correlations between predominant IPMN-B types and pathological diagnoses, with consideration of presenting symptoms and serum concentrations of tumor markers. Thus, the clinical, pathological, and trans-abdominal ultrasound findings of 20 patients with IPMN-B were reviewed. To our knowledge, this study has the largest number of IPMN-B patients who underwent conventional trans-abdominal ultrasound examination, with a review of the complete results. The average age of our patients was 60 years, with a similar number of men and women. This is consistent with previous reports (8,9).
IPMN-B is an uncommon biliary tumor, recently recognized, that manifests as an intraductal papillary tumor with mucin hyper-secretion. It is pathophysiologically similar to IPMN of the pancreas (4,10). Studies by Lim et al. (6,8) described four radiological imaging types of biliary dilation caused by IPMN-B, and our present study included 20 cases of IPMN-B with reference to that classification. Combined with the TAU features we determined, we stratified the 20 cases as types I–IV, that is, non-tumor, asymmetrical, symmetrical, and aneurismal dilation, respectively. We then compared the clinical and pathological data of each type.
Our findings showed that the most common clinical presentation was abdominal pain, occurring in 14 of the 20 patients (70%). This is similar to previous reports (11,12). The next most common symptoms were jaundice and fever, which are related to cholangitis and obstruction of the biliary tract caused by huge amounts of mucin. However, these manifestations are similar to many other kinds of biliary disease, including recurrent pyogenic cholangitis caused by bile duct stone, cholangiocarcinoma, and MCN. Since the clinical features of IPMN-B lack specificity, for many patients the time from onset of first self-conscious symptom to diagnosis was prolonged.
In this series of 20 cases, there were 3 of adenocarcinoma with no symptoms, comprising one with type II with only mild dilation of the common bile duct (1.4 cm), and 2 with type IV without any bile duct dilation. This indicates that asymptomatic cases of IPMN-B disease require careful attention and examination.
We also reviewed serum levels of CA19-9, CEA, and AFP in 12 of the 20 cases, and elevated CA19-9 or CEA was detected only when malignancy was present. This may be valuable for the diagnosis of malignancy.
Pathology of the surgical specimens revealed that all 20 cases had intraluminal masses of the bile duct, with large amounts of mucin. Most of our cases had been originally diagnosed as MCN, prior to the recognition of IPMN-B as a specific entity by Zen et al. (4) in 2006. The earliest presentation in the current study was 2002. Compared to IPMN of the pancreas, the pathological diagnosis of malignancy in IPMN-B was much higher reached 75% (9), which is similar to the result of this study. The preferred recommendation for treatment of IPMN-B is surgical resection. Therefore, an accurate preoperative diagnosis is important.
Conventional trans-abdominal ultrasound is usually the first choice for abdominal imaging examination. Ultrasound is especially sensitive for biliary disease with bile duct dilation, and it can be performed under any condition without invasion or radiation exposure. Our research showed that ultrasound could clearly show the biliary dilation caused by IPMN-B. Unlike a pathology report, ultrasound could correctly locate the position of the involved lobe caused by IPMN-B, and the region and the degree of bile duct expansion. In previous studies, the involved lobe was usually the left (10,11), which is consistent with our findings here.
However, in the present study, in three cases of type I on ultrasound, masses were not detected, but only diffuse remarkable dilation of both the intrahepatic and extrahepatic bile duct. In these cases, small, flat, and superficially spread intraductal papillary tumors were revealed macroscopically. The pathological results showed that all of these cases were benign, and the border intraluminal tumors functioned to secrete large amounts of mucin. Therefore, it is possible to suggest a diagnosis of benign IPMN-B for a patient with significant dilation of the intrahepatic and extrahepatic bile duct that has no visible obstruction. On the other hand, all of the five cases of type IV were malignant adenocarcinoma with local bile duct dilation and solid nodules within it due to early invasion into bile duct and the mucin retention, which may on behalf of poorly differentiated.
Due to the lack of specific clinical features and rarity, IPMN-B is difficult to diagnose accurately before surgery. Trans-abdominal ultrasound should be the first choice for imaging examination, and the characteristics of IPMN-B on trans-abdominal ultrasound images should be kept in mind to differentiate it from similar diseases such as biliary MCN, recurrent pyogenic cholangitis, and mass-forming cholangiocarcinoma (8,13,14). Biliary MCN is a closed sac, and while the intra-sac tumor secretes mucin, the mucin is confined and does not usually communicate with the biliary tree. Diffuse proximal and distal dilation of the bile duct will not show on ultrasound images. Even if the bile duct is obstructed by the large MCN sac, only the proximal dilation of the bile duct may be revealed.
Recurrent pyogenic cholangitis caused by bile duct stone is the most difficult to differentiate from IPMN-B. Images of the former may also show diffuse biliary dilation and intraductal masses (15), and mucin plugs are usually confused with stone on ultrasound images. Therefore, a multimodality assessment via different imaging techniques and clinical experience is necessary.
Regarding mass-forming type of cholangiocarcinoma, hypersecretion of mucin causes biliary dilation both upstream and downstream. However, in IPMN-B, ultrasound images will show a single solid or cystic mass, with only the upstream bile duct dilated due to obstruction.
In conclusion, IPMN-B is a rare and newly recognized biliary disease, and a good understanding of its clinical, imaging, and pathological features is needed. Based on the present review, an elevation of serum tumor markers may indicate a malignancy. The most typical trans-abdominal ultrasound feature of IPMN-B was significant diffuse intrahepatic and extrahepatic bile duct dilation, especially for benign cases. Some malignant cases only showed serious local bile duct dilation with intraductal papillary neoplasm. Conventional trans-abdominal ultrasound sensitively shows bile duct dilation. The images should be carefully evaluated for useful diagnostic clues, especially for asymmetric significant diffuse biliary dilation due to intraductal tumors of IPMN-B. Our report is expected to contribute to the accumulative and progressive understanding of this new disease entity (16). A possible diagnosis of IPMN-B should be suspected when there is significant dilation of the bile duct, after exclusion of extrahepatic bile duct obstructive disease.
Acknowledgments
Funding: This study was supported by the grants from the CAMS Innovation Fund for Medical Sciences (CIFMS, 2016-I2M-3-005).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yì-Xiáng J. Wáng, Yong Wang) for the series “Translational Imaging in Cancer Patient Care” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.09.33). The series “Translational Imaging in Cancer Patient Care” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All procedures performed in studies were in accordance with the ethical standards of The Institutional Review Board (IRB) of Peking Union Medical College Hospital (PUMCH) (No. S-K324). Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li ZS, Li Q. The latest 2010 WHO classification of tumors of digestive system. Zhonghua Bing Li Xue Za Zhi 2011;40:351-4. [PubMed]
- Ohashi K, Murakami Y, Murayama M, et al. Four cases of ‘‘mucin-producing’’ cancer of the pancreas on specific findings of papilla of Vater. Prog Dig Endosc 1982;20:348-51.
- D’souza MA, Isaksson B, Löhr M, et al. The clinicopathological spectrum and management of intraductal papillary mucinous neoplasm of the bile duct (IPMN-B). Scand J Gastroenterol 2013;48:473-9. [Crossref] [PubMed]
- Zen Y, Fujii T, Itatsu K, et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology 2006;44:1333-43. [Crossref] [PubMed]
- Tsuyuguchi T, Sakai Y, Sugiyama H, et al. Endoscopic diagnosis of intraductal papillary mucinous neoplasm of the bile duct. J Hepatobiliary Pancreat Sci 2010;17:230-5. [Crossref] [PubMed]
- Lim JH, Jang KT, Choi D. Biliary intraductal papillary-mucinous neoplasm manifesting only as dilatation of the hepatic lobar or segmental bile ducts: imaging features in six patients. AJR Am J Roentgenol 2008;191:778-82. [Crossref] [PubMed]
- Guo J, Liu Z, Sun S, et al. Biliary intraductal papillary-mucinous neoplasm in the left hepatic lobe diagnosed by endoscopic ultrasonography: Report of a case. Endosc Ultrasound 2016;5:274-5. [Crossref] [PubMed]
- Lim JH, Yoon KH, Kim SH, et al. Intraductal papillary mucinous tumor of the bile ducts. Radiographics 2004;24:53-66; discussion 66-7. [Crossref] [PubMed]
- Yeh TS, Tseng JH, Chiu CT, et al. Cholangiographic spectrum of intraductal papillary mucinous neoplasm of the bile ducts. Ann Surg 2006;244:248-53. [Crossref] [PubMed]
- Barton JG, Barrett DA, Maricevich MA, et al. Intraductal papillary mucinous neoplasm of the biliary tract: a real disease? HPB (Oxford) 2009;11:684-91. [Crossref] [PubMed]
- Minagawa N, Sato N, Mori Y, et al. A comparison between intraductal papillary neoplasms of the biliary tract (BT-IPMNs) and intraductal papillary mucinous neoplasms of the pancreas (P-IPMNs) reveals distinct clinical manifestations and outcomes. Eur J Surg Oncol 2013;39:554-8. [Crossref] [PubMed]
- Paik KY, Heo JS, Choi SH, et al. Intraductal papillary neoplasm of the bile ducts: the clinical features and surgical outcome of 25 cases. J Surg Oncol 2008;97:508-12. [Crossref] [PubMed]
- Ohtsuka M, Kimura F, Shimizu H, et al. Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion. Am J Surg Pathol 2011;35:512-21. [Crossref] [PubMed]
- Weiss KE, Willmann JK, Jeffrey RB, et al. Secondary sclerosing cholangitis in a critically ill patient. Quant Imaging Med Surg 2016;6:224-8. [Crossref] [PubMed]
- Yang J, Wang W, Yan L. The clinicopathological features of intraductal papillary neoplasms of the bile duct in a Chinese population. Dig Liver Dis 2012;44:251-6. [Crossref] [PubMed]
- Wáng YX. Systemic review and meta-analysis of diagnostic imaging technologies. Quant Imaging Med Surg 2016;6:615-8. [Crossref] [PubMed]


