
Autophagy blockade enhances the anti-cancer effect of Romidepsin in gastric cancer
Introduction
Gastric cancer (GC) is an aggressive disease that remains the third leading cause of mortality worldwide (1). Surgery is the main method for treating GC, but the results are unsatisfactory (2,3). One reason for this is that GC is often diagnosed at an advanced stage, especially in China (4). Therefore, there is a need for more effective therapies for advanced GC.
Histone deacetylase inhibitors (HDACis) exhibit synergistic anticancer effects with many other anticancer reagents, which suggest that combining HDACis with other antitumor agents may be an attractive therapeutic strategy for using these agents (5). Romidepsin, a Food and Drug Administration-approved HDACi, acts as an anticancer agent (6). We previously reported that romidepsin acts as a potential anticancer agent in hepatocellular carcinoma (7).
Autophagy is stimulated during various pathological states, for example when cells are exposed to chemotherapeutic agents (8,9). Autophagy functions as a pro-survival pathway that helps tumor cells resist apoptosis triggered by chemotherapeutic agents (10). The process of autophagy involves multiple steps including initiation, nucleation, elongation, closure, maturation and degradation (11). The biogenesis of autophagy requires a variety of proteins, including LC3-I/II, ATG and beclin-1 (12).
In the present study, we demonstrate that romidepsin induced autophagy in GC cells by regulating the extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR) signaling pathways in vitro and in vivo (7). Inhibiting autophagy with hydroxychloroquine (HCQ) significantly augmented romidepsin-induced apoptosis in GC cells. Thus, our results suggest that combining romidepsin and HCQ may represent a novel therapeutic strategy in GC.
Methods
Reagents
Romidepsin (FK228, C24H36N4O6S2), 3-methyladenine (3-MA) and HCQ were purchased from Selleck Chemicals (Houston, TX, USA). The BCA protein assay and annexin V–fluorescein isothiocyanate apoptosis detection kits were purchased from KeyGen Biotech (Nanjing, China). Annexin and 4',6-diamidino-2-phenylindole (DAPI) were purchased from GuGe Biotech Co. Ltd. (Wuhan, China). Methanol and ethanol were purchased from Shanghai LingFeng Chemical Reagent Co. Ltd. (Shanghai, China). Dimethyl sulfoxide (DMSO), Tween 20 and glycine were purchased from Sangon Biotech Co. Ltd. (Shanghai, China). Skim milk was purchased from Becton, Dickinson and Company (San Diego, CA, USA). Primary antibodies against LC3 A/B (LC3), p62 (also known as SQSTM1), beclin-1, ATG7, (c)-caspase-3, c-caspase-9, c-poly (ADP-ribose) polymerase (c-PARP), phosphorylated (p)-ERK, ERK, p-mTOR and mTOR were purchased from Cell Signaling Technology (Danvers, MA, USA); Anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody was purchased from Abcam (Cambridge, MA, USA).
Cells and animal model
The MGC-803 and BGC-823 cell lines were obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences, Shanghai Institute of Cell Biology. MGC-803 and BGC-823 cells were cultured in minimum essential medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum.
Animal experiments were performed in accordance with National Institutes of Health guidelines. Cells (2×106) were re-suspended in 100 µL phosphate-buffered saline, and injected subcutaneously into the lateral flanks of immunodeficient mice. After 5 days, the mice were assigned to groups treated with DMSO (control), romidepsin (0.5 mg/kg), HCQ (60 mg/kg) or romidepsin + HCQ. Romidepsin and DMSO were administered by intraperitoneal injection once every 3 days for 21 days. HCQ was dissolved in saline solution and administered by intraperitoneal injection daily. Tumor volume and body weight were measured every 4 days for 21 days. After 20 days, tumors were harvested. Tumor volumes (V) were calculated using the following equation: V (cm3) = width2 (cm2) × length (cm)/2.
Transmission electron microscopy (TEM)
After being treated with romidepsin or DMSO for the indicated time, MGC-803 cells were collected and fixed with 2.5% phosphate-buffered glutaraldehyde. The fixed cells were stained with 1% osmium tetroxide in a buffer solution, and then dehydrated using an ascending ethanol and acetone gradient. Finally, the cells were embedded in epoxy resin and photographed with a Philips TECNAI 10 transmission electron microscope.
Western blot assay
Briefly, after treatment with romidepsin or DMSO for the indicated time, GC cells were lysed. Protein concentrations were quantified by Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions. After denaturation, the proteins were separated by gel electrophoresis using 12% SDS-PAGE and transferred electrophoretically to polyvinylidene fluoride membranes. The membranes were incubated in blocking buffer for 2 h, then at 4 °C with primary antibody, then finally at 37 °C for 2 h with the appropriate secondary antibody.
CCK8, flow cytometric analysis, tumor histology and immunohistochemistry
Procedures and reagents were as previously described (7).
Statistical analysis
All statistical analyses were conducted using SPSS 22.0 software. Data are presented as mean ± SD error of the mean of 3 independent experiments. P values were derived from 2-sided tests. P<0.05 was considered statistically significant.
Results
Romidepsin induced autophagy in GC cells
Autophagy can be activated in cells exposed to HDACis, including romidepsin. To investigate the effect of romidepsin on autophagy in GC, we examined several autophagy-related proteins. LC3, beclin-1 and ATG7 are upregulated during autophagy, and the autophagic substrate p62 is degraded. We found that romidepsin induced the conversion of LC3-I to LC3-II (Figure 1A), and decreased p62 levels in a similar manner (Figure 1A). Moreover, romidepsin treatment led to a significant increase in beclin-1 and ATG7 levels (Figure 1A). These findings suggest that romidepsin promotes the initiation of autophagy.
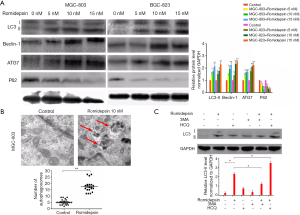
To confirm the initiation of autophagy, we used TEM to check for autophagosome formation in romidepsin-treated cells. This showed that romidepsin led to the accumulation of autophagosomes, visible as double-membraned structures containing organelle remnants, whereas only a few autophagosomes were observed in control cells (Figure 1B).
Autophagy can be blocked by 3-MA, which inhibits phosphoinositide 3-kinase and lysosome function. We used 3-MA to investigate romidepsin-induced autophagy in GC cells. Romidepsin treatment increased LC3-II levels, but this increase was attenuated in the presence of 3-MA (Figure 1C).
Taken together, these findings indicate that as expected, romidepsin induced autophagy.
Romidepsin may induce autophagy via the ERK/mTOR pathway
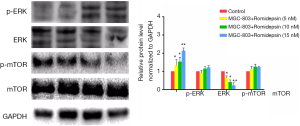
Previously, we reported that MAPK signaling pathway components (such as ERK, p38 and JNK) are involved in cell cycle regulation. Furthermore, the mTOR and ERK pathways are known regulators of autophagy in mammalian cells. To investigate whether these signaling pathways are involved in the romidepsin-mediated induction of autophagy in GC, western blot analysis was used to evaluate ERK and mTOR activation in GC cells. Consistent with previous reports, romidepsin treatment resulted in increased concentrations of p-ERK (Figure 2). Meanwhile, romidepsin inhibited the phosphorylation of mTOR kinase (Ser2448). These results suggest that romidepsin-induced autophagy in GC cells may be mediated by activation of the ERK and mTOR pathways.
Blocking autophagy enhances the anticancer effect of romidepsin in GC cells
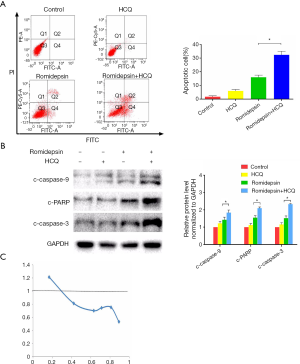
Appropriate modification of autophagy might enhance the anticancer therapeutic efficacy of HDACis. To investigate the role of autophagy in romidepsin-induced cytotoxicity, GC cells were pre-treated with HCQ for 4 h before treatment with romidepsin for 24 h. HCQ prevented an increase in the number of apoptotic cells; this result was confirmed by western blotting and flow cytometric analysis (Figure 3A,B). The combination index was employed to investigate whether romidepsin and HCQ act synergistically (Figure 3C) (13). The results suggest that HCQ significantly enhanced the anticancer effect of romidepsin in GC cells.
Inhibiting autophagy enhances the anticancer effects of romidepsin in vivo
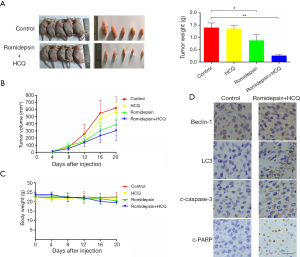
We next investigated whether a combination of romidepsin and HCQ would synergistically induce GC cell death in vivo. MGC-803 cells were subcutaneously injected into nude mice to further probe the tumor-suppressive effect of the combination of romidepsin and HCQ in vivo. The nude mice were treated with romidepsin (0.5 mg/kg) or DMSO (control) intraperitoneally once every 3 days for 21 days. HCQ (60 mg/kg) was administered daily thereafter. HCQ alone did not have a substantial effect on tumor growth, whereas HCQ in combination with romidepsin significantly reduced tumor growth compared with romidepsin alone (Figure 4A). The results showed there was no significant loss in body weight in the combination treatment group (Figure 4B,C). Immunohistochemical staining of xenograft tissues revealed higher beclin-1 and LC3 levels in the combination treatment group (Figure 4D). Moreover, c-PARP and c-caspase-3 levels were much higher in the combination treatment group (Figure 4D). Thus, inhibiting autophagy enhanced the anticancer effects of romidepsin.
Discussion
Because many cases are diagnosed at an advanced stage, GC has high incidence and mortality in China (14). Therefore, more effective therapies for advanced GC are required (15). Several studies have shown that autophagy inhibition sensitizes tumor cells to HDACi-induced apoptosis (5,16). Targeting autophagy is therefore an attractive potential strategy for cancer therapy (8). Hui et al. demonstrated that a combination of bortezomib and romidepsin could potently induce GC cell death through a mechanism involving caspase-independent autophagy (17). However, to date, no studies have explored whether romidepsin alone induces autophagy in GC cells. Here, we demonstrated that romidepsin induced autophagy in GC. We observed that autophagy was cytoprotective during romidepsin-induced apoptosis. Furthermore, we showed that inhibiting autophagy with HCQ sensitized GC cells to romidepsin-induced apoptosis.
Autophagy is stimulated by various stress conditions, for example when cells are exposed to chemotherapeutic agents (18). Autophagy functions as a pro-survival pathway that helps tumor cells resist apoptosis triggered by chemotherapeutic agents (12). The process of autophagy involves multiple steps, including initiation, nucleation, elongation, closure, maturation and degradation (18). The biogenesis of autophagy requires a variety of proteins, including LC3-I/II, ATG and beclin-1. Our results showed that LC3-I was converted to LC3-II in GC cells after romidepsin exposure. Moreover, western blot assays revealed that beclin-1 and ATG7 levels synchronously increased in romidepsin-treated cells. Romidepsin-treated cells exhibited punctate LC3-II staining characteristic of autophagy, visualized by confocal microscopy. p62, which is a major autophagy substrate, is degraded after autophagosome–lysosome fusion. We found that romidepsin decreased p62 expression in a dose-dependent manner. TEM, a classic autophagy detection method, further revealed the vesicular structures were double-membraned vesicles, termed autophagosomes, which engulf intracellular components (19).
The ERK signaling pathway plays an essential role in cell autophagy (20), but a link between this pathway and the mechanism of action of HDACis has not been identified (21). The mTOR pathway also plays important roles in regulating autophagy. Evidence has been reported to suggest that inhibition of mTOR activity may be the link between ERK in several human cell lines (22). Thus, we analyzed levels of phosphorylated ERK and mTOR in romidepsin-treated GC cells. The results clearly showed that romidepsin inhibited mTOR phosphorylation and activated the ERK pathway. Our findings suggest that romidepsin activates autophagy, possibly by activating the ERK and mTOR pathways.
Since the realization that autophagy plays essential roles in GC cells, there has been great interest in inhibiting autophagy in combination with antitumor agent use for GC therapy (23). HCQ, which blocks lysosome function, is a clinically relevant autophagy inhibitor and is being widely assessed clinically (5,24). We performed median effect analysis to investigate whether romidepsin and HCQ act synergistically (13). The combination index tended towards values of less than one, indicating the combination was synergistic. Our results clearly show pre-treatment with HCQ strongly enhanced romidepsin-mediated apoptosis in GC in vivo and in vitro.
In conclusion, our results revealed that romidepsin induced autophagy in GC cells, possibly by regulating the ERK and mTOR signaling pathways. Notably, our study demonstrates combining HCQ with romidepsin may represent a novel chemotherapeutic strategy in GC, and possibly other types of solid cancers.
Acknowledgments
Funding: This work was supported by Innovative Research Groups of the National Natural Science Foundation of China (No. 81421062), the Major program of National Natural Science Foundation of China (No.91542205), the National S&T Major Project of China (No. 2012ZX10002017), Zhejiang Provincial Natural Science Foundation of China (LY18H160046), and Zhejiang Medical Science Foundation (2018KY532).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.09.51). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived. The animal study was approved by the First Affiliated Hospital of Zhejiang University School of Medicine.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang L, Yin J, Wang X, et al. C-Type Lectin-Like Receptor 2 Suppresses AKT Signaling and Invasive Activities of Gastric Cancer Cells by Blocking Expression of Phosphoinositide 3-Kinase Subunits. Gastroenterology 2016;150:1183-95. e16.
- Mirkin KA, Luke FE, Gangi A, et al. Sarcopenia related to neoadjuvant chemotherapy and perioperative outcomes in resected gastric cancer: a multi-institutional analysis. J Gastrointest Oncol 2017;8:589-95. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Zheng HL, Lu J, Li P, et al. Effects of Preoperative Malnutrition on Short- and Long-Term Outcomes of Patients with Gastric Cancer: Can We Do Better? Ann Surg Oncol 2017;24:3376-85. [Crossref] [PubMed]
- Mahalingam D, Mita M, Sarantopoulos J, et al. Combined autophagy and HDAC inhibition: a phase I safety, tolerability, pharmacokinetic, and pharmacodynamic analysis of hydroxychloroquine in combination with the HDAC inhibitor vorinostat in patients with advanced solid tumors. Autophagy 2014;10:1403-14. [Crossref] [PubMed]
- Saijo K, Imamura J, Narita K, et al. Biochemical, biological and structural properties of romidepsin (FK228) and its analogs as novel HDAC/PI3K dual inhibitors. Cancer Sci 2015;106:208-15. [Crossref] [PubMed]
- Sun WJ, Huang H, He B, et al. Romidepsin induces G2/M phase arrest via Erk/cdc25C/cdc2/cyclinB pathway and apoptosis induction through JNK/c-Jun/caspase3 pathway in hepatocellular carcinoma cells. Biochem Pharmacol 2017;127:90-100. [Crossref] [PubMed]
- Ruocco N, Costantini S, Costantini M. Blue-Print Autophagy: Potential for Cancer Treatment. Mar Drugs 2016;14:E138 [Crossref] [PubMed]
- Ruan Y, Hu K, Chen H. Autophagy inhibition enhances isorhamnetininduced mitochondriadependent apoptosis in nonsmall cell lung cancer cells. Mol Med Rep 2015;12:5796-806. [Crossref] [PubMed]
- Anding AL, Baehrecke EH. Autophagy in Cell Life and Cell Death. Curr Top Dev Biol 2015;114:67-91. [Crossref] [PubMed]
- Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 2012;11:709-30. [Crossref] [PubMed]
- White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer 2012;12:401-10. [Crossref] [PubMed]
- Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006;58:621-81. [Crossref] [PubMed]
- Nie Y, Wu K, Yu J, et al. A global burden of gastric cancer: the major impact of China. Expert Rev Gastroenterol Hepatol 2017;11:651-61. [Crossref] [PubMed]
- Xu L, Pan Q, Lin R. Prevalence rate and influencing factors of preoperative anxiety and depression in gastric cancer patients in China: Preliminary study. J Int Med Res 2016;44:377-88. [Crossref] [PubMed]
- Lachenmayer A, Toffanin S, Cabellos L, et al. Combination therapy for hepatocellular carcinoma: additive preclinical efficacy of the HDAC inhibitor panobinostat with sorafenib. J Hepatol 2012;56:1343-50. [Crossref] [PubMed]
- Hui KF, Yeung PL, Chiang AK. Induction of MAPK- and ROS-dependent autophagy and apoptosis in gastric carcinoma by combination of romidepsin and bortezomib. Oncotarget 2016;7:4454-67. [Crossref] [PubMed]
- Rebecca VW, Amaravadi RK. Emerging strategies to effectively target autophagy in cancer. Oncogene 2016;35:1-11. [Crossref] [PubMed]
- Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016;12:1-222.
- Dillon LM, Bean JR, Yang W, et al. P-REX1 creates a positive feedback loop to activate growth factor receptor, PI3K/AKT and MEK/ERK signaling in breast cancer. Oncogene 2015;34:3968-76. [Crossref] [PubMed]
- Basu S, Rajakaruna S, Reyes B, et al. Suppression of MAPK/JNK-MTORC1 signaling leads to premature loss of organelles and nuclei by autophagy during terminal differentiation of lens fiber cells. Autophagy 2014;10:1193-211. [Crossref] [PubMed]
- Ugland H, Naderi S, Brech A, et al. cAMP induces autophagy via a novel pathway involving ERK, cyclin E and Beclin 1. Autophagy 2011;7:1199-211. [Crossref] [PubMed]
- Qian HR, Yang Y. Functional role of autophagy in gastric cancer. Oncotarget 2016;7:17641-51. [Crossref] [PubMed]
- Yoshida GJ. Therapeutic strategies of drug repositioning targeting autophagy to induce cancer cell death: from pathophysiology to treatment. J Hematol Oncol 2017;10:67. [Crossref] [PubMed]


