
Quantification of solid hypo-echoic thyroid nodule enhancement with contrast-enhanced ultrasound
Introduction
In current clinical practice, doctors typically prefer ultrasound examination as the imaging modality to evaluating, assessing, and monitoring thyroid nodule problems. Currently, several ultrasonography (US) characteristics have been proposed to assist in the diagnosis of malignant thyroid nodules. Six US characteristics (1,2), including a predominantly solid composition, hypo-echogenicity, irregular margins, absence of a halo, microcalcifications, and an anteroposterior (AP) diameter larger than the axial diameter (taller than wider shape), could be potential predictors of thyroid malignancy. Endorsed by the Society of Radiologists in an Ultrasound Consensus Conference statement (3), the above US features, especially solid consistency and hypo-echogenicity, are highly relevant to thyroid cancer. Papini et al. reported that the combination of a solid echostructure with a hypo-echogenic pattern has 87% sensitivity but low specificity and positive predictive value (PPV) for diagnosing thyroid cancer (4). Gul et al. also found that 99.1% of thyroid cancers were solid and that 86.6% appeared as hypo-echoic nodules on conventional ultrasound (CUS). However, the specificities of these factors were poor given that 47.7–75.9% of benign nodules were solid and up to 55% of benign nodules were hypo-echoic (5). Therefore, preoperative discrimination of solid hypo-echoic thyroid nodules (SHTNs) remains challenging, and the characteristics of SHTNs on US are important in clinical practice.
Fine needle aspiration biopsy (FNAB) is currently widely used in the diagnosis of thyroid nodules and has the highest specificity (72–100%) (6,7). However, this technique may have some weaknesses given its poor sensitivity (65–98%). Approximately 10–20% of thyroid nodules could not be diagnosed, 1–2% were false negative results, 3–16% were non-diagnostic and 6–20% exhibited indeterminate (follicular lesions) outcomes in previous studies (7-9). These indeterminate lesions are associated with a risk of malignancy of approximately 25% (10,11). However, some patients refuse to undergo FNAB; therefore, other effective ultrasound examinations are needed for the diagnosis of benign and malignant thyroid nodules.
Contrast-enhanced ultrasound (CEUS) is a new modality for the diagnosis of thyroid nodules (12). CEUS provides a better representation of the vascular pattern. Some authors have reported that ring enhancement is more likely to indicate a benign lesion, whereas heterogeneous enhancement perfusion curves characterize malignant nodules (13-15). However, the role of CEUS in the thyroid remains controversial, and the vascular enhancement pattern for solid SHTNs is unknown. Hence, the present study sought to evaluate the diagnostic performance of CEUS for the differential diagnosis of benign and malignant SHTNs.
Methods
Patients and selection criteria
Between October 2014 and April 2016, a total of 97 patients with 123 nodules were examined with CEUS after detection by CUS. The inclusion criteria were (I) solid nodules; (II) hypo-echogenicity on US classified with the Thyroid Imaging Reporting and Data System as categories 3–5; (III) no macrocalcification (large calcifications in the thyroid nodules result in perfusion defects regardless of whether the nodule is benign or malignant) and (IV) verification by pathological examination after surgical resection. Among 123 nodules, 31 nodules with no pathology results and 5 nodules with unsatisfactory CEUS video clips were not included in this study. Thus, the pathology results for 87 nodules in 77 patients (mean age, 52.4±17.2 years; range, 19–84 years) were obtained after surgery. The study was approved by the Clinic Institutional Review Board (IRB) of Huadong Hospital, Fudan University. Informed consent was obtained from all patients.
Conventional US and CEUS examinations
CUS and CEUS examinations were performed with a Siemens Acuson S2000 US instrument (Siemens, Mountain View, CA, USA), and a 5- to 14-MHz linear array transducer (9L4, Siemens Medical Solutions) was used in all examinations. Thyroid nodules were assessed for size, location, shape, margin, echogenicity presence/absence of halo sign, and presence/absence of microcalcification or macrocalcification. After a B-mode ultrasound examination, color Doppler US was performed to evaluate intranodular vascularity.
The maximum plane that included the whole lesion and its surrounding normal tissue was selected for CEUS. CEUS was performed under a low mechanical index between 0.06 and 0.10 with a gain of 100–120 dB and a frame rate of 26. The focus was set below the target lesion, and the time gain compensation was positioned on the midline. The parameters remained the same during the examination. The contrast agent used in this study was SonoVue (Bracco, Milan, Italy). Contrast-tuned imaging and microflow imaging software were incorporated in the system and used here. In total, 1.5 mL SonoVue was administered via a peripheral vein in a bolus followed by a 5-mL normal saline flush. Continuous imaging was performed immediately after injection of the contrast agent and lasted for 3 min. For multiple nodules, if one section could not be simultaneously observed, two contrast agent injections were performed.
Image analysis
The thyroid nodules were evaluated on CEUS relative to a normal thyroid parenchyma. The enhancement degree was classified as hyper-, iso-, or hypo-enhancement and assessed relative to the surrounding normal thyroid tissue at the peak time. The microbubble arrival time/degradation time was classified as earlier, at the same time, or later. Peripheral enhancement was classified as existent or none. Peripheral enhancement was defined as enhancement occurring exclusively or predominantly at the periphery of the lesion. Enhancement homogeneity was classified as homogeneous or heterogeneous. Homogeneous enhancement was defined as uniform enhancement throughout the lesion. Heterogeneous enhancement was defined as non-uniform enhancement exhibiting variations within the lesion. During the post-processing analysis, the operator manually drew a region of interest (ROI) covering the tissue to be studied, and a color map was automatically generated by the system. Then, further and smaller ROIs of similar size were hand-drawn on the color map within the tumor. Finally, the peak of enhancement [Peak (%)], time to peak [Tp (s)], area under the curve [AUC (%/s)], and mean transit time [MTT (s)] for each ROI were automatically measured by the system.
The examinations were performed by one radiologist who had 9 years of experience in thyroid US and 3 years of experience in thyroid CEUS. Retrospective analyses of contrast-enhancement patterns and kinetic data were performed by two radiologists not involved in the US scanning and blinded to the final diagnosis. If a disagreement occurred, another radiologist reviewed the image until a consensus was reached.
Statistical analysis
Statistical analyses were performed using SPSS software version 19.0 (make). The sensitivity, specificity, PPV, negative predictive value (NPV), and diagnostic accuracy were calculated by comparing the findings with the results of histological reports. Continuous quantitative data were expressed as the mean ± standard deviation (SD). Conventional US characteristics and CEUS enhancement features of thyroid nodules were performed using the χ2 test. Student’s t-test was used to compare the CEUS parameters of both benign and malignant thyroid nodules. A value of P<0.05 was considered to indicate a significant difference.
Results
Histopathologic analysis
Among the 87 thyroid nodules that were histology confirmed, a single nodule was detected in 62 patients, and multiple nodules were noted in 15 patients. Forty-seven nodules were found on the right side, 33 were found on the left side, and 7 were located in the thyroid isthmus. The mean diameter of the thyroid nodules was 13.5±5.3 mm with a range from 5 to 33 mm. Of the 87 nodules, 32 (36.8%) were benign (23 nodule goiters, 8 follicular adenomas, and 1 granuloma), and 55 (63.2%) were malignant (53 papillary carcinomas and 2 follicular carcinomas).
Conventional US features of SHTNs
The mean maximum diameters of the thyroid nodules obtained from CUS were 13.7±6.2 mm (5 to 33 mm) for benign lesions and 12.1±5.3 mm (6 to 30 mm) for malignant lesions (P>0.05). Significant differences were found for the indicators, including halo, microcalcifications, orientation and intranodular vascularity, on CUS between benign and malignant lesions. However, no significant differences in the shape, margin and homogeneity were noted. Table 1 details the image features of benign and malignant nodules on CUS. Predominantly, solid composition, hypo-echogenicity, irregular margins, absence of a halo, microcalcifications, a taller rather than wider shape, and intranodular vascularization were considered suspicious features of malignancy in CUS.
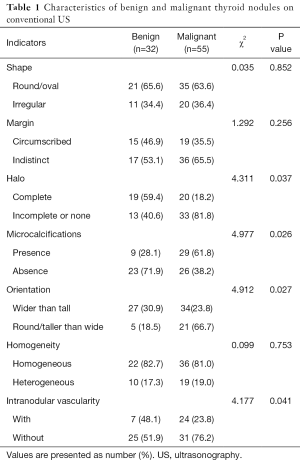
Full table
Enhancement features
No adverse events or side effects were recorded during or immediately after the injection of SonoVue. Benign and malignant thyroid nodules exhibited different enhancement features (Table 2). The 55 malignant nodules exhibited mainly absent or faint dotted enhancement patterns including absence in 9 (16.36%), hypo-enhancement in 41 (74.55%), and hyper-enhancement in 5 (0.91%) nodules. The 32 benign thyroid nodules primarily exhibited diffuse enhancement, including hyper-enhancement in 18 (56.25%), iso-enhancement in 6 (18.75%), hypo-enhancement in 5 (15.63%), and an absence of enhancement in 3 nodules (0.94%) (Figure 1). In the qualitative evaluations, the Peak and AUC of most malignant nodules were reduced compared to those of the surrounding normal thyroid tissue. In addition, the Tp and MTT were also delayed. In most benign nodules, the Peak, Tp, AUC and MTT were similar to those of surrounding normal thyroid tissue. The Peak, AUC, enhancement degree, and enhancement homogeneity on CEUS were significantly different between benign and malignant nodules (P<0.05) (Table 3; Figures 2-5).
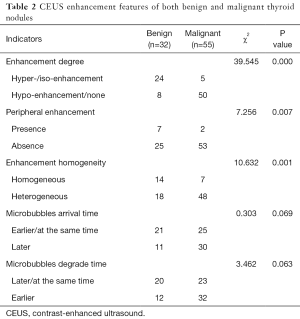
Full table
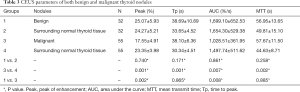
Full table
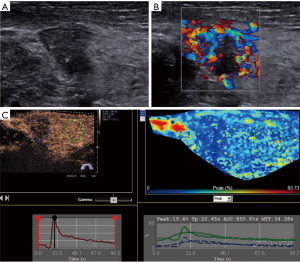
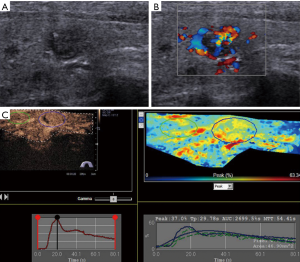
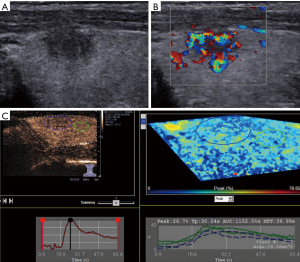
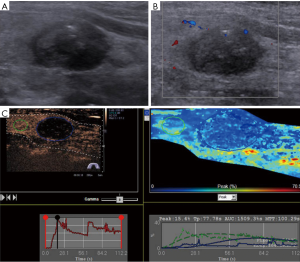
Comparison of the diagnostic value of CEUS and CUS
The diagnostic performance of the two different modalities for discrimination between benign and malignant thyroid lesions is presented in Table 4. Of the 87 nodules, 51 were diagnosed as malignant, and 26 were benign on CEUS. In addition, 10 nodules were misdiagnosed. With regard to CUS, 42 nodules were suspicious for malignancy, and 21 were likely benign. In total, 24 nodules were misclassified (Figures 4,5). Although no significant differences were noted between CEUS and CUS with respect to specificity (81.3% vs. 65.6%), PPV (89.5% vs. 79.2%) and false positive rate (18.8% vs. 34.4%), these methods differed significantly in terms of sensitivity (92.7% vs. 76.4%), NPV (86.7% vs. 61.8%), false negative rate (7.3% vs. 23.6%) and accuracy (87.4% vs. 72.4%).
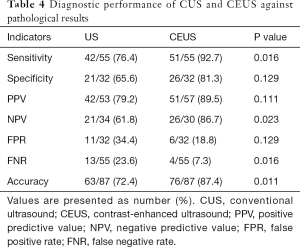
Full table
Discussion
Up to 68% of people have thyroid nodules. Most thyroid malignancies are solid and hypo-echoic on CUS. The rate of malignancy of SHTNs is higher in all thyroid nodules (10–13%) (16,17). Thus, a precise evaluation of SHTNs via clinical examination is very important and can avoid unnecessary surgery on patients. FNAB is currently the primary diagnostic procedure and has varying sensitivity, but not all patients are suitable for FNAB (6-9). Therefore, another effective method to accurately evaluate thyroid nodules is needed.
Currently, CEUS is considered a relatively safe technique and does not involve ionizing radiation or the risk of nephrotoxicity (18,19). CEUS has the capability to clearly display microvascular blood flow in tumors, and it can accurately evaluate the sequence and intensity of tumor perfusion and vascularity. Several studies have observed specific CEUS enhancements in different types and stages of thyroid nodules; ring-like enhancement was observed in benign lesions, and heterogeneous enhancement was observed in malignant lesions. Consistent with this finding, several previous studies have also found that CEUS can accurately distinguish benign lesions from malignant lesions. Unfortunately, not all studies have confirmed these results (15,20,21). Bartolotta et al. (20) assessed the enhanced features related to the size of the lesion rather than histology and found that nodules measuring less than 1 cm exhibited mainly absent vascularization. In addition, nodules with a diameter of 1 to 2 cm revealed faint dotted contrast enhancement. Nodules with a diameter larger than 2 cm presented diffuse contrast enhancement. Few studies thus far have focused on the utility of CEUS for SHTNs. To comprehensively illustrate this issue, the present study used CEUS to evaluate the enhanced appearance of SHTNs.
Our study showed that CEUS outperformed CUS for diagnosing SHTNs, as noted in Tables 2,4. This study showed that the majority of solid hypo-echoic malignant nodules appeared mostly heterogeneous with hypoenhancement or even no enhancement, whereas the solid hypo-echoic benign lesions were homogeneous with hyperenhancement or iso-enhancement. This characteristic may be related to the vasculature of thyroid nodules. Tissue fibrosis of the thyroid gland due to blood vessels of malignant nodules is typically aberrant and tortuous. Ring enhancement and homogeneity of the enhancement of thyroid nodules on CEUS is correlated with a benign lesion (14). However, in this study, only seven benign nodules exhibited peripheral ring enhancement, which was significantly lower than the rate reported in other studies. This difference may account for the pathological type of nodules, and most of the lesions were nodular goiters.
Based on the result that hypo-enhancement was considered a malignancy, the sensitivity, specificity, PPV, NPV, false positive rate, false negative rate and accuracy were 92.7%, 81.3%, 89.5%, 86.7%, 18.8%, 7.3% and 87.4%, respectively. Regarding CUS, the sensitivity, specificity, PPV, NPV, false positive rate, false negative rate and accuracy were 76.4%, 65.6%, 79.2%, 61.8%, 34.4%, 23.6% and 72.4%, respectively, based on the US characteristics presented in Table 1. CEUS significantly outperformed CUS in differentiating SHTNs. Our results were consistent with prior studies by other authors who reported that CEUS can provide useful additional information to differentiate thyroid nodules (21). The quantitative analysis of CEUS yielded a sensitivity of 76.9%, a specificity of 84.8% and an accuracy of 82.6% for differentiating thyroid nodules. Another study further confirmed that CEUS is a promising noninvasive technique for the differential diagnosis of benign and malignant thyroid nodules and could be a valuable supplemental method to FNAB (15). In this study, the quantitative analysis of CEUS provides additional useful information (Table 3). The Peak and AUC indicated the intensity of enhancement. Tp and MTT were related to the time of enhancement. For the malignant tumors, the Peak, Tp, AUC and MTT were 17.55%, 38.10 s, 1,028.51%/s and 57.67 s, respectively, whereas these values were 23.35%, 30.34 s, 1,497.74%/s and 44.63 s for the surrounding parenchyma. A statistically significant difference between tumor and parenchyma was noted. The Peak, Tp, AUC and MTT observed for the benign tumors and the surrounding parenchyma were not significantly different. In contrast, the Peak and AUC of malignant nodules were markedly decreased compared to those of benign lesions. This finding suggests that malignant nodules lacked a blood supply, whereas benign nodules were characterized by relatively rich blood perfusion. This finding may be attributable to the differences in the microvascular density and intranodular pressure. However, Tp and MTT did not correspond to significant differences. Although a rapid Tp and MTT might be expected in malignancy, we found no significant difference in the Tp and MTT measurements between benign and malignant lesions. Similar results have been demonstrated by other studies, which highlights the overlap in the vascularization of carcinomas and adenomas (22,23).
In conclusion, our results strongly support the high diagnostic value of CEUS for the differential diagnosis of benign and malignant tumors in SHTNs. CEUS may be a promising diagnostic technique for the diagnosis of thyroid nodules in the future. However, further studies with more samples are required to more strongly support our findings. A limitation of this study was that we did not observe a correlation between contrast-enhanced sonography and microvessel density (MVD) in thyroid nodules. If MTT could be reversely correlated with MVD, the diagnostic value of CEUS in thyroid nodules would be further confirmed.
Acknowledgments
Funding: This work was supported by grant No. 14411970400 from the Medical Guide Project of the Shanghai Science and Technology Commission.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yì-Xiáng J. Wáng, Yong Wang) for the series “Translational Imaging in Cancer Patient Care” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.09.15). The series “Translational Imaging in Cancer Patient Care” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Clinic Institutional Review Board (IRB) of Huadong Hospital, Fudan University and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Woliński K, Szkudlarek M, Szczepanek-Parulska E, et al. Usefulness of different ultrasound features of malignancy in predicting the type of thyroid lesions: a meta-analysis of prospective studies. Pol Arch Med Wewn 2014;124:97-104. [PubMed]
- Remonti LR, Kramer CK, Leitão CB, et al. Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid 2015;25:538-50. [Crossref] [PubMed]
- Frates MC, Benson CB, Charboneau JW, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Ultrasound Q 2006;22:231-8; discussion 239-40. [Crossref] [PubMed]
- Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab 2002;87:1941-6. [Crossref] [PubMed]
- Gul K, Ersoy R, Dirikoc A, et al. Ultrasonographic evaluation of thyroid nodules: comparison of ultrasonographic, cytological, and histopathological findings. Endocrine 2009;36:464-72. [Crossref] [PubMed]
- Isaac A, Jeffery CC, Seikaly H, et al. Predictors of non-diagnostic cytology in surgeon-performed ultrasound guided fine needle aspiration of thyroid nodules. J Otolaryngol Head Neck Surg 2014;43:48. [Crossref] [PubMed]
- Al-azawi D, Mann GB, Judson RT, et al. Endocrine surgeon-performed US guided thyroid FNAC is accurate and efficient. World J Surg 2012;36:1947-52. [Crossref] [PubMed]
- Bohacek L, Milas M, Mitchell J, et al. Diagnostic accuracy of surgeon-performed ultrasound-guided fine-needle aspiration of thyroid nodules. Ann Surg Oncol 2012;19:45-51. [Crossref] [PubMed]
- Nou E, Kwong N, Alexander LK, et al. Determination of the optimal time interval for repeat evaluation after a benign thyroid nodule aspiration. J Clin Endocrinol Metab 2014;99:510-6. [Crossref] [PubMed]
- Yoo WS, Choi HS, Cho SW, et al. The role of ultrasound findings in the management of thyroid nodules with atypia or follicular lesions of undetermined significance. Clin Endocrinol (Oxf) 2014;80:735-42. [Crossref] [PubMed]
- Rago T, Scutari M, Latrofa F, et al. The large majority of 1520 patients with indeterminate thyroid nodule at cytology have a favorable outcome, and a clinical risk score has a high negative predictive value for a more cumbersome cancer disease. J Clin Endocrinol Metab 2014;99:3700-7. [Crossref] [PubMed]
- Yu D, Han Y, Chen T. Contrast-enhanced ultrasound for differentiation of benign and malignant thyroid lesions: meta-analysis. Otolaryngol Head Neck Surg 2014;151:909-15. [Crossref] [PubMed]
- Zhang B, Jiang YX, Liu JB, et al. Utility of contrast-enhanced ultrasound for evaluation of thyroid nodules. Thyroid 2010;20:51-7. [Crossref] [PubMed]
- Ma JJ, Ding H, Xu BH, et al. Diagnostic performances of various gray-scale, color Doppler, and contrast-enhanced ultrasonography findings in predicting malignant thyroid nodules. Thyroid 2014;24:355-63. [Crossref] [PubMed]
- Hornung M, Jung EM, Georgieva M, et al. Detection of microvascularization of thyroid carcinomas using linear high resolution contrast-enhanced ultrasonography (CEUS). Clin Hemorheol Microcirc 2012;52:197-203. [PubMed]
- Asteria C, Giovanardi A, Pizzocaro A, et al. US-elastography in the differential diagnosis of benign and malignant thyroid nodules. Thyroid 2008;18:523-31. [Crossref] [PubMed]
- Nam-Goong IS, Kim HY, Gong G, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf) 2004;60:21-8. [Crossref] [PubMed]
- Quaia E. Assessment of tissue perfusion by contrast-enhanced ultrasound. Eur Radiol 2011;21:604-15. [Crossref] [PubMed]
- Rafaelsen SR, Jakobsen A. Contrast-enhanced ultrasound vs multidetector-computed tomography for detecting liver metastases in colorectal cancer: a prospective, blinded, patient-by-patient analysis. Colorectal Dis 2011;13:420-5. [Crossref] [PubMed]
- Bartolotta TV, Midiri M, Galia M, et al. Qualitative and quantitative evaluation of solitary thyroid nodules with contrast-enhanced ultrasound: initial results. Eur Radiol 2006;16:2234-41. [Crossref] [PubMed]
- Nemec U, Nemec SF, Novotny C, et al. Quantitative evaluation of contrast-enhanced ultrasound after intravenous administration of a microbubble contrast agent for differentiation of benign and malignant thyroid nodules: assessment of diagnostic accuracy. Eur Radiol 2012;22:1357-65. [Crossref] [PubMed]
- Argalia G, De Bernardis S, Mariani D, et al. Ultrasonographic contrast agent: evaluation of time-intensity curves in the characterisation of solitary thyroid nodules. Radiol Med 2002;103:407-13. [PubMed]
- Appetecchia M, Bacaro D, Brigida R, et al. Second generation ultrasonographic contrast agents in the diagnosis of neoplastic thyroid nodules. J Exp Clin Cancer Res 2006;25:325-30. [PubMed]


