
Exosomes in pancreatic juice as valuable source of biomarkers for early diagnosis of pancreatic cancer
Introduction
Despite increasing use of advanced imaging technology in cancer diagnosis, there remains a pressing need for early diagnosis in order to improve survival outcomes. This is particularly pertinent in the field of pancreatic cancer (PC), for which survival outcomes remain remarkably dire. In fact, current projections predict that only lung cancer will surpass PC as the number one killer of all cancers in the United States within the next two decades (1). The lethality of PC is largely attributable to the insidious nature of disease onset and progression until symptoms appear and patients present to health care systems. By the time patients are symptomatic, only 20% are viable candidates for surgery (2). Even with surgical resection, survival outcomes remain unfavourable (3). Despite the advent of new treatments in recent years, the 5-year survival rate remains low at approximately 8%. Alarmingly, the mortality rate and incidence rate of PC continue to be closely aligned (4).
Pancreatic juice has been emerging over the past decade as a promising source of potential biomarkers that can detect PC in asymptomatic patients. Previous studies have illustrated the ability for pancreatic juice samples, rather than surgically resected PC tissue, to be used for determining molecular profiles indicative of PC (5). Molecular profiling of potential PCs is particularly important, since different classes of pancreatic masses have different malignant and pre-malignant potential (6). Of particular interest are masses with pre-malignant potential, since these may be where the greatest potential for early and successful therapeutic intervention lies.
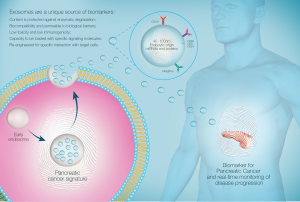
In order for therapeutic intervention to have maximal success, it is essential to diagnose pancreatic tumor as early as possible and thereby restrict the tumors’ malignant potential. In order to achieve this, recent studies have focused on molecular methods of diagnosing early pancreatic lesions in the form of biomarkers. One particularly promising source of biomarkers is extracellular vesicles (EVs), which have recently come to the forefront of biomarker research as a key mediator of cell-cell communication (7). Amongst the range of EVs, exosomes are particularly exciting and have been the subject of intense research over the course of the past decade. Exosomes are highly stable, membrane bound vesicles which are released from all cells in the body, including cells from diseased tissue as in cancer. Exosomes are distinguished by a number of features, namely their size (40–100 nm) and the endocytic pathway of biogenesis. Given their endosomal origin, exosomes encapsulate a unique host of molecular cargo which is trafficked between the tissue of origin to contiguous organs. This is particularly notable in the context of cancer, in which tumor exosomes have been suggested to traffic molecular cargo from the initial tumor lesion to distal sites, thus mediating metastatic cellular communication (8). This includes a host of information including a vast array of proteins, miRNA, and mRNA (7). Therefore, it is critical that exosomes carry molecular information that is specific to the cell of origin. In cases where the cell of origin is inflicted with pathology, as in the case of cancer, we suggest that exosomes transfer hallmarks of cancer pathology to contiguous organs and distal sites to initiate metastasis. As such, this mechanism of cellular communication may be crucial in the diagnosis of PC in the early stages. Importantly, past studies have highlighted the stability of exosomes and their cargo in a wide range of biological fluids (9,10). Through the analysis of exosomes in pancreatic juice, we may be able to trace the pre-metastatic potential of early pancreatic lesions. Additionally, this may facilitate the development of increasingly accurate prognoses for early lesions in order to guide management. Hence, this review aims to firstly outline the current knowledge and common methods used in the diagnosis of PC, followed by emerging data in biomarker research for PC. In particular, there will be a focus on the abundance of information and unique biomarkers identified in pancreatic juice. We discuss how the diagnostic potential of pancreatic juice can be enhanced through the analysis of pancreatic juice-derived exosomes.
PC diagnosis
The diagnosis of PC is notoriously difficult, with the majority of patients presenting with vague symptoms such as pain and weight loss. In later stages, jaundice may also present prior to diagnosis. These symptoms arise due to a mass effect, leading to interruption of normal pancreatic function (11). However, by this point the majority of tumors are already deemed non-resectable, owing to their rapid metastasis and ready invasion of surrounding vessels particularly in the posterior region of the pancreas (11). This is a major contributor the dismal survival rate of PC.
Notably, while tumors in the head of the pancreas may present in the early stages with signs of biliary obstruction, it is tumors in the pancreatic body and tail that are the most insidious and are commonly found at an advanced stage (12). PC stems from precursor lesions called pancreatic intraepithelial neoplasias which are <5 mm and difficult to detect with traditional imaging modalities (13).
Although widely available, abdominal ultrasounds are not commonly used for effective evaluation of pancreatic etiologies. Abdominal ultrasounds are useful in investigating initial presentations of jaundice and abdominal pain through attempts to find biliary dilation. However, accuracy of ultrasounds in the diagnosis of pancreatic tumors is only 50–70% (14).
Meanwhile, computerised tomography (CT) scans have contributed to advances in pancreatic tumor diagnosis and staging throughout the past decade. While non-contrast CT scans are not able to image pancreatic tumors with high sensitivity or specificity, CT with intravenous contrast provides higher quality images. In particular, multidetector CT (MDCT) is able to increase resolution by compiling several thin slices. This enables the visualisation of the mass relative to important anatomical landmarks such the superior mesenteric artery and vein and the portal vein (15). Consequently, MDCT with intravenous contrast has been deemed an important component of the initial diagnostic workup (12).
Currently, EUS-guided fine needle aspiration (EUS-FNA) is one of the mainstays of PC diagnosis, particularly in situations where suspected cancer cannot be located using the aforementioned imaging modalities (12). EUS-FNA is particularly advantageous in its ability to provide direct visualization of a variety of lesions in the mediastinal and intra-abdominal regions (16). Beyond visualization, EUS-FNA is a method for attaining cytological samples hence further facilitating accurate diagnosis and staging of pancreatic lesions (17).
Despite the often incomplete information provided by imaging modalities for diagnosis of PC, early detection of PC remains crucial in altering the prognosis of the patient. Intraductal papillary mucinous neoplasms (IPMN), a subtype of PC, have a relatively high chance of being cured with surgical resection, but only if there has not been any infiltration of an adenocarcinoma (18). Pre-invasive intraepithelial neoplasias at the head of the gland are a precursor for adenocarcinoma, and thus crucial to identify to avoid infiltration (19). Given that their size is <5 mm, imaging modalities are not yet a reliable way to identify early pancreatic lesions prior to infiltration (20).
Some biomarkers have emerged over the past decade in an attempt to overcome the shortcomings of traditional imaging. It has been suggested that cancer antigen 19-9 (CA19-9) may be able to accurately diagnose malignant tumors (21). However, the recent body of research has demonstrated that the use of CA19-9 in diagnostics is limited with inadequate sensitivity. The sensitivity and specificity can vary widely, but can often be as low as 41% and 33% respectively (22). This is largely because CA19-9 levels are high even in benign pancreatic disease. Conversely, CA19-9 levels may often be low in early pre-malignant or malignant pancreatic masses (23). Carcinoembryonic antigen (CEA) is currently a standard marker for colorectal cancer, although originally aimed to diagnose PC. It is hypothesised that malignant cells express increased CEA, since it has a role in cell adhesion (24). However, further studies on the use of CEA as a diagnostic biomarker of PC have been underwhelming. Results of a meta-analysis highlighted that in PDAC, the median sensitivity was 54% and median specificity was 79%. In addition, due to high association with other cancers, CEA is often not able to specifically diagnose PDAC (25).
Due to these limitations, very few PC patients are identified early enough to be benefited by current treatments. More definitively diagnostic biomarkers which are informative in the early stages are required in order to improve patient outcomes.
The importance of pancreatic juice in the diagnosis of PC
One of the many difficulties of diagnosing and assessing PC is the retroperitoneal location of the pancreas. This limits accessibility and complicates visualization. However, several studies suggest that pancreatic juice may serve as a surrogate means for investigating pathologies, such as chronic pancreatitis or PC (26).
Pancreatic juice has come to attention over the past decade as a potentially useful source of diagnostic aids. It can be extracted using multiple methods, including aspiration under duodenoscopy, pancreatic juice aspiration with or without secretin stimulation, or aspiration with percutaneous puncture using ultrasonography (27,28).
Dalbec et al. performed an endoscopic retrograde cholangiopancreatography on patients with various pathological diagnoses. ELISA was then performed to determine the presence and expression of pro-inflammatory molecules such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-alpha), and monocyte chemoattractant protein-1 (MCP-1). Hence, this study established that pancreatic juice provides a unique snapshot of the inflammatory environment in the pancreas. Notably, this inflammatory milieu has been implicated in PC pathogenesis (26).
A further study by Yamaguchi et al. suggests the use of a brushing catheter to access proliferations in the main pancreatic duct, enabling the retrieval of neoplastic cells with potentially greater sensitivity (29). However, this retrospective study found that the sensitivity of the pancreatic juice cytology in detecting IPMNs was only 40%. Researchers suggest the difficulties of using pancreatic juice cytology to guide diagnosis may arise from the distance between the lesion and the ampulla of Vater or the main pancreatic duct, as well as potentially impaired exocrine function. Furthermore, it was found that even in areas of malignancy, neoplastic cells appeared well differentiated (29).
Given the low sensitivity of cytological investigations using pancreatic juice, some studies have turned to microRNA (miRNA) and proteins as a way to diagnose PC at an earlier stage.
The proteomic profile of pancreatic juice may provide a unique reflection of the functional status of the pancreas. A study by Doyle et al. investigated the proteome of non-pathological pancreatic juice. A total of 285 proteins were detected from the fluid, with a majority of these involved in cellular functions such as proteolysis, lipid metabolism, anion exchange, or DNA and RNA function. Interestingly, the study also compared the proteome of normal pancreatic juice with published data on proteins found in samples from patients with PC. The comparison revealed an overlap of only 42 proteins, with tumor markers such as CEA, mucin 1, and azurocidin only found in cancer samples (30). It is reported that pancreatic juice may provide a source of PC specific proteins, as cancer cells are shed into the ductal lumen (31). Gao et al. analysed pancreatic juice samples from patients with PC, comparing this to samples from chronic pancreatitis and choledocholithiasis patients. PC cell lines were found to have a significantly higher expression of serine proteinase-2 (PRSS2) mRNA (31). PRSS2 has a putative role in tumor progression through extracellular matrix degradation and matrix metalloproteinase activation (32). Among other changes, there was also an upregulation of pancreatic lipase-related protein (PLRP1) and downregulation of precursors for chymotrypsinogen B (CTRB) and elastase 3B (ELA3B), the latter of which is used as an indicator of pancreatic function (31,33).
Furthermore, alterations in the miRNA profile have also been detected. miRNAs are 19–25 nucleotides in length, representing non-coding RNAs. They are important in the regulation of gene expression. Given this role, some miRNAs have been implicated in oncogenesis and abnormal tissue differentiation in various cancers, including PC (34,35). It is reported that these alterations in the miRNA profile may be reflected in pancreatic juice. A study by Sadakari et al. established the presence of miRNAs in pancreatic juice (36). The study also sought to identify particular miRNAs in pancreatic juice that could then be used to identify PDAC. It was found that miRNA-21 and miRNA-155 were expressed at significantly higher levels in pancreatic tissue and juice samples from patients with PDAC, compared to patients with chronic pancreatitis (36). miRNA-155 has previously been noted to be overexpressed in IPMN (37). miRNA-21 is also apparently overexpressed in PC cells compared to chronic pancreatitis (38). In terms of functionality, previous studies have shown that miRNA-21 increases MMP2, MMP9 and VEGF expression to hence enhance cellular proliferation and invasion (39). Meanwhile miRNA-155 has previously been associated with lymphoma cell activation, as well as lymphocyte regulation (40). Recent reports have found that in PC in particular, miRNA-155 may be implicated in the pathological conversion of fibroblasts to cancer-associated fibroblasts (41). This illustrates that disparities in the expression of miRNA between cancerous and non-cancerous conditions may bear functional consequences related to the normal cellular roles of particular miRNAs. Interestingly, while expression levels of these two miRNAs were remarkable, cytological analysis of the samples did not reflect these changes. This further reinforces that molecular analysis of pancreatic juice may be superior to cytological analysis in differentiating PC from chronic pancreatitis or the norm.
It has also been suggested that genetic alterations may be revealed through analysis of pancreatic juice. Matsubayashi et al. investigated methylation-specific PCR assays to identify abnormal methylation of DNA in patients with suspected PC, as well as suspected chronic pancreatitis or normal controls undergoing screening due to familial risk factors for PC (42). A significantly higher proportion of patients with PC compared to patients without neoplasias had increased methylation. Patients with chronic pancreatitis also had a lower abnormal methylation level compared to PC patients or those at risk of PC (42). Hence, examining genetic changes in pancreatic juice potentially provides a further tool for investigating suspected PC or pre-invasive neoplasms before lesions become apparent on imaging.
Genetic changes in pancreatic juice of PC patients were further studied by Eshleman et al. (43) Researchers analysed pancreatic juice from patients undergoing EUS for suspected PC. KRAS mutations were found to a greater extent in pancreatic juice samples from patients with PC or suspected to have PC compared to controls without suspected PC. Similarly, a meta-analysis by Yang et al. assessed several studies to demonstrate that KRAS mutations may be diagnostically valuable for PC (44). This indicates that pancreatic juice may contain genetic information to guide PC diagnosis and prognosis in the early stage, even when abnormalities are not detected on imaging (43). However, previous studies have noted that when looking at KRAS mutations in whole pancreatic juice, sensitivity and specificity may not be sufficient for practical, clinical relevance (44). Particular subcellular compartments within pancreatic juice may provide a more specific source of cancer-associated molecular changes.
Given that pancreatic juice is relatively easy to retrieve with multiple available techniques, there is potential for the emerging field of biomarkers to expand and take advantage of this unique snapshot of the cellular environment. EVs may provide a way to circumvent the issue of low sensitivity and specificity of some forms of pancreatic juice analysis. The current body of literature suggest EVs, particularly exosomes, contain a set of molecular cargo which aids and identifies cancer progression and metastasis.
Exosomes: a new key to unlocking the potential of pancreatic juice
Over the last decade, exosomes have emerged as an important mechanism of cell-cell communication. Exosomes are a subtype of EVs which have featured prominently at the forefront of biomarker research across a range of disease processes, including pregnancy-associated pathologies, a number of cancers and nervous system cellular communication (45-47). Exosomes are notable amongst other EVs for a number of features. The first distinctive feature is their size. Exosomes are 40–100 nm. On the other hand, microvesicles are approximately 50–1,000 nm while apoptotic bodies range between 800–5,000 nm (7). More biologically notable is the unique cellular pathway from which exosomes are derived. Exosomes are derived from multivesicular bodies (MVBs), which constitute particular endosome compartments. The MVBs bud inwardly, hence fusing with the plasma membrane and releasing their cargo of exosomes into the extracellular compartment through the exocytotic pathway (48,49). This endocytic origin is particularly notable as it attributes exosomes with molecular components from the cell of origin. This includes proteins such as TSG101 CD81, and CD63, as well as miRNA. The diverse exosomal cargo also includes a variety of signalling molecules, such as growth factor receptors and cell adhesion molecules. While the pathways by which exosomes are packed with their molecular cargo are yet to be fully understood, it is known that this is an enzyme and ATP dependent process. Additionally, RAB27A and RAB27B activity have been identified as important in promoting exosome secretion (50). Other studies have found that the tumor suppressor p53 may upregulate exosome synthesis. Additionally, the interaction of syndecan with syntenin alongside ALIX through specific amino acid motifs promotes inward budding of the exosomes during biogenesis (51,52). Further research is required to fully appreciate and integrate the numerous molecular regulators which govern exosome synthesis and production.
It is well known that exosomes are able to travel in all body fluids, including blood, urine and milk (53). Exosomes may be trafficked through body fluids to either neighbouring cells or distant organs to regulate their function through the molecular data packaged within. At the target site, signalling receptors of target cells interact with transmembrane proteins on the surface of exosomes. Following this, there is fusion with target cell plasma membranes and release of content into the cytoplasm of the recipient cell (54).
This is important in the context of tumor progression and metastasis. Exosomes and other types of EVs offer tumors a unique mode of paracrine signalling. This establishes a pathway for tumor growth and metastasis (55). Using nanoparticle tracking analysis, Balaj et al. found that tumor cells can release between approximately 7,000 and 25,000 microvesicles per cell per 48 hours, compared to 3,800–6,200 microvesicles per cell per 48 hours in normal, non-pathological fibroblasts (56). Additionally, a study by Muller et al. established that exosomes are a key mediator of cell-cell communication especially in cancer tissue (57). Notably, exosomes were isolated at consistently higher levels from cancer samples, compared to non-cancerous controls (57). Exosomes and other EVs are hence hypothesized to be a way for tumor cells to crosstalk with the surrounding tissue. This further illustrates the need to investigate exosomes within pancreatic juice.
Exosomes hence provide a signature of the cell of origin and are a shuttle for unique molecules that progress the cancer micro-environment. As such, exosomes in pancreatic juice may provide a refined selection of clinical biomarkers for PC.
Diagnostic utility of exosomes in pancreatic juice
Proteins associated with exosomes
Proteins have featured prominently in recent literature as potential clinical biomarkers for various cancers, particularly PC. A wide array of proteins are packaged into exosomes, including transport proteins, fusion proteins, and proteins required for biogenesis of EVs, for example TSG101 and ALIX (58). Although containing a wide range of proteins, it is important to note that exosomal proteins are specific for the cell-type of origin and particular cellular signalling pathways (58). Several studies have highlighted that based on this selectivity, exosomes may carry proteins that alter the face of the cellular micro-environment. As such, this micro-environment can be directed towards a pro-oncogenic state through the transfer of exosomal protein (59,60).
This phenomenon is demonstrated by the finding that PC progression is promoted by pancreatic cancer initiating cells (PACICs). These cells are marked by surface proteins, including CD44v6, CD133, MET, and tetraspanin 8 (Tspan8). Wang et al. found that these surface markers are packed and delivered through PC-derived exosomes (61). Exosomes bearing these markers were later found to promote metastasis of tumor cells to the lungs and lymph nodes. In particular, Jung et al. described exosomal CD44v as having an important role in this process. CD44v promotes tumor metastasis through establishing a soluble matrix (62). More recently, Yue et al. found that exosomal Tspan8 in addition to exosomal CD151 facilitated matrix degradation and stromal reprogramming in the rat pancreatic adenocarcinoma line. In addition, these exosomal components promoted non-metastatic cells to become more motile and directed cells towards secondary sites through the inclusion and activation of integrins (63).
There has been extensive interest in understanding how PDAC metastasizes to critical secondary sites, such as the liver. Supporting earlier research, Costa-Silva et al. further reinforced the idea that exosomes carry proteomic cargo that induces metastasis in PDAC. There was a heightened predisposition to metastasis when PDAC-derived exosomes were transferred to the livers of naive mice. Following engulfment by Kupffer cells, exosomes were able to induce the formation of a fibrotic microenvironment (8). Additional research has found that a key feature of this fibrotic microenvironment which promotes metastasis is the increased concentration of macrophage migration inhibitory factor (MIF). MIF is an established pro-inflammatory cytokine. In addition, it is hypothesized that MIF may facilitate angiogenesis and proliferation in tumors (64). MIF has also been reported to induce epithelial to mesenchymal transition (EMT). This transition leads to loss of cell polarity and adhesion, thus increasing the migratory capacity of cells (65). Exosomal-MIF has been suggested as a biomarker to indicate PDAC metastasis to the liver, especially since MIF is highly expressed in cases of hepatic PDAC metastases (65).
Another feature of the fibrotic microenvironment which promotes PC metastasis is heightened levels of myofibroblasts which contribute to the formation of collagen-rich scar tissue. Myofibroblasts lead to alteration of the architecture of the surrounding tissue and extracellular matrix. In addition, there are further stromal changes which promote angiogenesis and cellular invasive and proliferative capacity (66). Webber et al. demonstrated that through increased surface expression of TGF-β and β-glycan, exosomes were able to trigger the production of fibroblast growth factor 2 (FGF2). In turn, PC-associated exosomes promoted the conversion of fibroblasts to myofibroblasts.
Another feature that may also promote PC progression is regulation of exosome synthesis and secretion. RAB27A is a Rab GTPase which is required during vesicle transportation (67). Tumor protein 53 (TP53) is another protein which is required for exosome secretion (52). Interestingly, both RAB27A and TP53 levels have been positively correlated with clinical features seen in PC. A study by Wang et al. found that with increased RAB27A expression, there was heightened vascularization and tumor invasiveness (68). Increases in both RAB27A and TP53 were associated with increasingly dire prognoses for patient survival.
Several other changes in the proteomic profile of PC-derived exosomes have been identified in the literature. Indeed, a study by Adamczyk et al. was able to identify 3,000 proteins in exosomes associated with PC. Of note in this secretome was the epidermal growth factor receptor (EGFR) (69). Binding of EGFR has been associated with increased activation of carcinogenesis signal transduction. This is consistent with the finding that EGF and TGF-α, which are ligands of EGFR, are overexpressed in the majority of PC subtypes (70). Increased binding of EGFR has been associated with increased tumor invasiveness and proliferation, thus aiding metastasis and cellular migration (71). A study by Arscott et al. further supports the notion that exosomal EGFR can be used as a biomarker for PC, as cancer-associated exosomes contained high concentrations of EGFR (72). In addition, PC-derived exosomes were found to have elevated membrane associated proteins, GTP-binding proteins, and glycoproteins compared to exosomes derived from normal controls (73).
Hence, it is important to further study exosomes as a diagnostic biomarker for PC progression as exosomes may provide an early indicator of cellular differentiation of benign cells to malignant cells.
miRNAs associated with exosomes
Aside from proteins, exosomes are also a valuable source of miRNAs. Exosomes were first described to contain miRNA by Valadi et al., with later reports demonstrating that the trafficking of miRNA by exosomes led to particular biological outcomes in the target cell following exocytosis of the exosomal cargo (41,74). It has been suggested that the transfer of miRNA by exosomes allows the gradual formation of a premetastatic niche.
It is known that the inflammatory response is important in the progression of PC (26). Some recent studies have suggested that exosomes may carry cargo to secondary sites of metastasis, thus promoting the inflammatory response. A study by Fabbri et al. found that tumors secrete exosomes which contain miRNA that can later bind to Toll-like receptors (TLRs) in immune cells, thereby upregulating the inflammatory process (75). This contributes to an increased proliferative capacity of the primary tumor. This further highlights the role of exosomal miRNA in promoting a pro-metastatic cellular environment.
A study by Rana et al. further illustrated this phenomenon using the rat pancreatic adenocarcinoma cell line (BSp73ASML) (76). These cells are known to highly express CD44 v4-v7, which promote metastasis. Researchers hypothesized that exosomes from Bsp73ASMLwt would allow cross-talk between tumor cells and target cells, thus contributing to metastasis. The study examined the miRNA and mRNA profile contained within exosomes from tumor cells. Results illustrated that miRNA was packaged into the BSp73ASML exosomes. Furthermore, CD44v was identified as having a modulatory role on the mRNA and miRNA profile contained within exosomes. The miRNAs of note in this study were miRNA-494 and miRNA-542-3p. These were found to selectively regulate mRNA in order to promote the formation of a premetastatic niche. Hence it was established that transfer of exosomal miRNA plays a direct role in shaping the genetic information in target cells (76).
Notably, changes in the miRNA profile of pancreatic juice have been reflected in miRNA profile alterations in EVs derived from other fluids. As mentioned, pancreatic juice from patients with PC has been shown to contain higher expression of certain miRNAs, such as miRNA-155 (36). Pang et al. found that microvesicles which contain higher levels of miRNA-155 can promote the conversion of normal pancreatic fibroblasts to cancer-associated fibroblasts, which is an important step in tumor progression. This conversion is thought to occur through the role of miRNA-155 in downregulating tumour protein p53-induced nuclear protein 1 (TP531NP1) (41). Since exosomes contain miRNA and proteins in a manner specific to the cell of origin, future studies should investigate other miRNAs with altered expression in pancreatic juice and whether these are altered within exosomes.
The differences in miRNA expression in exosomes in body fluid samples from patients with various cancers have been summarised previously, suggesting miRNA packaged within exosomes may be a promising biomarker to aid cancer diagnosis (77,78).
Ali et al. performed a comparison of miRNA expression in PC patients, compared to patients with chronic pancreatitis and healthy controls. Of note, miRNA-205, miRNA-155, and miRNA-31 were upregulated in patients with pancreatic tumors. Upregulation of these miRNAs was related to poorer survival and disease outcomes in patients with PC (79). This lends weight to the argument that miRNA in exosomes in body fluids of patients with cancer is related to oncogenesis. It also suggests identification of these miRNAs may be an important biomarker for disease status. However, the study by Ali et al. looked at tissue samples and patient plasma (79). Pancreatic juice may contain a higher proportion of tumor-derived exosomes due to preferential shedding of tumor cells into the ductal lumen (31). Thus, it is important to investigate exosomal miRNA within pancreatic juice.
A similar comparison was conducted recently by Madhavan et al. (80). This study involved a blind comparison between either exosome-depleted or exosome-enriched serum samples of patients with PC, as well as patients without PC. PC-derived exosomes were found to contain greater expression of several miRNAs, including miRNA-1246, miRNA-4644, miRNA-3976, and miRNA-4306. Notably, there was also a heightened expression of aforementioned cancer initiating cell markers (80). Further research is required to validate these biomarkers for use in the clinical context.
Conclusions
The study of exosomes within pancreatic juice and other biological fluids, rather than the fluid itself, is advantageous for multiple reasons. Firstly, exosome-rich samples have significantly higher miRNA content compared to supernatants depleted of exosomes. In addition, multiple miRNAs detected in exosomes could not be detected in serum or supernatant (81). Further studies have also found that whole plasma and serum contain comparatively less miRNA compared to samples enriched with exosomes (9). This suggests selective trafficking within EVs is an important route for transfer of miRNAs. Furthermore, exosomes are a stable source of miRNA (9). Indeed, exosomes are able to be stored for prolonged periods over several freeze-thaw cycles without significant alteration of their cargo (10). These are key features that may aid the development of clinically viable diagnostic biomarkers (Figure 1 and Table 1).
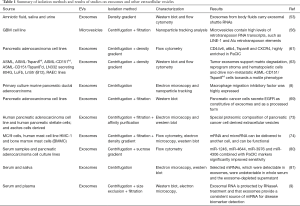
Full table
Acknowledgments
Funding: This review was supported by Lions Medical Research Foundation, UQ academic Title Holder Research Fund, and Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 1170809).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Edward R. Sauter) for the series “Body Fluid Exosomes and Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.10.21). The series “Body Fluid Exosomes and Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Simoes PK, Olson SH, Saldia A, et al. Epidemiology of pancreatic adenocarcinoma. Chin Clin Oncol 2017;6:24. [Crossref] [PubMed]
- Cinar P, Ko AH. Best practices for the treatment of metastatic pancreatic adenocarcinoma: the therapeutic landscape in 2017. Chin Clin Oncol 2017;6:29. [Crossref] [PubMed]
- NIH. Cancer Stat Facts: Pancreas Cancer. Surveillance, Epidemiology, and End Results Program. 2014.
- Rogers CD, Fukushima N, Sato N, et al. Differentiating pancreatic lesions by microarray and QPCR analysis of pancreatic juice RNAs. Cancer Biol Ther 2006;5:1383-9. [Crossref] [PubMed]
- Hibi Y, Fukushima N, Tsuchida A, et al. Pancreatic juice cytology and subclassification of intraductal papillary mucinous neoplasms of the pancreas. Pancreas 2007;34:197. [Crossref] [PubMed]
- Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014;30:255-89. [Crossref] [PubMed]
- Costa-Silva B, Aiello NM, Ocean AJ, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 2015;17:816-26. [Crossref] [PubMed]
- Cheng L, Sharples RA, Scicluna BJ, et al. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles 2014;3. [PubMed]
- Sarker S, Scholz-Romero K, Perez A, et al. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med 2014;12:204. [Crossref] [PubMed]
- Freelove R, Walling AD. Pancreatic cancer: diagnosis and management. Am Fam Physician 2006;73:485-92. [PubMed]
- Tummala P, Junaidi O, Agarwal B. Imaging of pancreatic cancer: An overview. J Gastrointest Oncol 2011;2:168-74. [PubMed]
- Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol 2001;25:579-86. [Crossref] [PubMed]
- Rickes S, Unkrodt K, Neye H, et al. Differentiation of pancreatic tumours by conventional ultrasound, unenhanced and echo-enhanced power Doppler sonography. Scand J Gastroenterol 2002;37:1313-20. [Crossref] [PubMed]
- Catalano C, Laghi A, Fraioli F, et al. Pancreatic carcinoma: the role of high-resolution multislice spiral CT in the diagnosis and assessment of resectability. Eur Radiol 2003;13:149-56. [PubMed]
- Weston BR, Bhutani MS. Optimizing Diagnostic Yield for EUS-Guided Sampling of Solid Pancreatic Lesions: A Technical Review. Gastroenterol Hepatol (N Y) 2013;9:352-63. [PubMed]
- Wang J, Wu X, Yin P, et al. Comparing endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) versus fine needle biopsy (FNB) in the diagnosis of solid lesions: study protocol for a randomized controlled trial. Trials 2016;17:198. [Crossref] [PubMed]
- Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology 2002;123:1500-7. [Crossref] [PubMed]
- Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 2004;28:977-87. [Crossref] [PubMed]
- Hanada K, Okazaki A, Hirano N, et al. Effective screening for early diagnosis of pancreatic cancer. Best Pract Res Clin Gastroenterol 2015;29:929-39. [Crossref] [PubMed]
- Datta J, Vollmer CM Jr. Investigational biomarkers for pancreatic adenocarcinoma: where do we stand? South Med J 2014;107:256-63. [Crossref] [PubMed]
- Bunger S, Laubert T, Roblick UJ, et al. Serum biomarkers for improved diagnostic of pancreatic cancer: a current overview. J Cancer Res Clin Oncol 2011;137:375-89. [Crossref] [PubMed]
- Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol 2007;33:266-70. [Crossref] [PubMed]
- Benchimol S, Fuks A, Jothy S, et al. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell 1989;57:327-34. [Crossref] [PubMed]
- Duraker N, Hot S, Polat Y, et al. CEA, CA 19-9, and CA 125 in the differential diagnosis of benign and malignant pancreatic diseases with or without jaundice. J Surg Oncol 2007;95:142-7. [Crossref] [PubMed]
- Dalbec KM, Max Schmidt C, Wade TE, et al. Adipokines and Cytokines in Human Pancreatic Juice: Unraveling the Local Pancreatic Inflammatory Milieu. Adipokines and Cytokines in Human Pancreatic Juice: Unraveling the Local Pancreatic Inflammatory Milieu. Dig Dis Sci 2010;55:2108-12. [PubMed]
- Nakaizumi A, Tatsuta M, Uehara H, et al. Usefulness of simple endoscopic aspiration cytology of pancreatic juice for diagnosis of early pancreatic neoplasm. A prospective study. Dig Dis Sci 1997;42:1796-803. [Crossref] [PubMed]
- Yamaguchi K, Ohuchida J, Ohtsuka T, et al. Intraductal papillary-mucinous tumor of the pancreas concomitant with ductal carcinoma of the pancreas. Pancreatology 2002;2:484-90. [Crossref] [PubMed]
- Yamaguchi K, Nakamura M, Shirahane K, et al. Pancreatic Juice Cytology in IPMN of the Pancreas. Pancreatology 2005;5:416-21; discussion 21. [Crossref] [PubMed]
- Doyle CJ, Yancey K, Pitt HA, et al. The proteome of normal pancreatic juice. Pancreas 2012;41:186. [Crossref] [PubMed]
- Gao J, Zhu F, Lv S, et al. Identification of pancreatic juice proteins as biomarkers of pancreatic cancer. Oncol Rep 2010;23:1683-92. [PubMed]
- Moilanen M, Sorsa T, Stenman M, et al. Tumor-associated trypsinogen-2 (trypsinogen-2) activates procollagenases (MMP-1, -8, -13) and stromelysin-1 (MMP-3) and degrades type I collagen. Biochemistry 2003;42:5414-20. [Crossref] [PubMed]
- Tian M, Cui YZ, Song GH, et al. Proteomic analysis identifies MMP-9, DJ-1 and A1BG as overexpressed proteins in pancreatic juice from pancreatic ductal adenocarcinoma patients. BMC Cancer 2008;8:241. [Crossref] [PubMed]
- Schultz NA, Werner J, Willenbrock H, et al. MicroRNA expression profiles associated with pancreatic adenocarcinoma and ampullary adenocarcinoma. Mod Pathol 2012;25:1609-22. [Crossref] [PubMed]
- Farazi TA, Hoell JI, Morozov P, et al. MicroRNAs in human cancer. Adv Exp Med Biol 2013;774:1-20. [Crossref] [PubMed]
- Sadakari Y, Ohtsuka T, Ohuchida K, et al. MicroRNA expression analyses in preoperative pancreatic juice samples of pancreatic ductal adenocarcinoma. JOP 2010;11:587-92. [PubMed]
- Habbe N, Koorstra JB, Mendell JT, et al. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol Ther 2009;8:340-6. [Crossref] [PubMed]
- Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA 2007;297:1901-8. [Crossref] [PubMed]
- Moriyama T, Ohuchida K, Mizumoto K, et al. MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresistance. Mol Cancer Ther 2009;8:1067-74. [Crossref] [PubMed]
- Chen DF, Gong BD, Xie Q, et al. MicroRNA155 is induced in activated CD4(+) T cells of TNBS-induced colitis in mice. World J Gastroenterol 2010;16:854-61. [PubMed]
- Pang W, Su J, Wang Y, et al. Pancreatic cancer-secreted miR-155 implicates in the conversion from normal fibroblasts to cancer-associated fibroblasts. Cancer Sci 2015;106:1362-9. [Crossref] [PubMed]
- Matsubayashi H, Canto M, Sato N, et al. DNA Methylation Alterations in the Pancreatic Juice of Patients with Suspected Pancreatic Disease. Cancer Res 2006;66:1208-17. [Crossref] [PubMed]
- Eshleman JR, Norris AL, Sadakari Y, et al. KRAS and Guanine Nucleotide-Binding Protein Mutations in Pancreatic Juice Collected From the Duodenum of Patients at High Risk for Neoplasia Undergoing Endoscopic Ultrasound. Clin Gastroenterol Hepatol 2015;13:963-9.e4. [Crossref] [PubMed]
- Yang J, Li S, Li J, et al. A meta-analysis of the diagnostic value of detecting K-ras mutation in pancreatic juice as a molecular marker for pancreatic cancer. Pancreatology 2016;16:605-14. [Crossref] [PubMed]
- Salomon C, Rice GE. Role of Exosomes in Placental Homeostasis and Pregnancy Disorders. Prog Mol Biol Transl Sci 2017;145:163-79. [Crossref] [PubMed]
- Brinton LT, Sloane HS, Kester M, et al. Formation and role of exosomes in cancer. Cell Mol Life Sci 2015;72:659-71. [Crossref] [PubMed]
- Zhang G, Yang P. A novel cell-cell communication mechanism in the nervous system: exosomes. J Neurosci Res 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Bang C, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol 2012;44:2060-4. [Crossref] [PubMed]
- Tkach M, Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016;164:1226-32. [Crossref] [PubMed]
- Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010;12:19-30; sup pp 1-13.
- Baietti MF, Zhang Z, Mortier E, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 2012;14:677-85. [Crossref] [PubMed]
- Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res 2006;66:4795-801. [Crossref] [PubMed]
- Keller S, Ridinger J, Rupp AK, et al. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med 2011;9:86. [Crossref] [PubMed]
- Tian T, Zhu YL, Hu FH, et al. Dynamics of exosome internalization and trafficking. J Cell Physiol 2013;228:1487-95. [Crossref] [PubMed]
- Friedman A, Hao W. The Role of Exosomes in Pancreatic Cancer Microenvironment. Bull Math Biol 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Balaj L, Lessard R, Dai L, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun 2011;2:180. [Crossref] [PubMed]
- Muller L, Hong CS, Stolz DB, et al. Isolation of biologically-active exosomes from human plasma. J Immunol Methods 2014;411:55-65. [Crossref] [PubMed]
- Choi DS, Kim DK, Kim YK, et al. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom Rev 2015;34:474-90. [Crossref] [PubMed]
- Blackwell RH, Foreman KE, Gupta GN. The Role of Cancer-Derived Exosomes in Tumorigenicity & Epithelial-to-Mesenchymal Transition. Cancers (Basel) 2017;9:E105 [Crossref] [PubMed]
- Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev 2013;32:623-42. [Crossref] [PubMed]
- Wang H, Rana S, Giese N, et al. Tspan8, CD44v6 and alpha6beta4 are biomarkers of migrating pancreatic cancer-initiating cells. Int J Cancer 2013;133:416-26. [Crossref] [PubMed]
- Jung T, Castellana D, Klingbeil P, et al. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia 2009;11:1093-105. [Crossref] [PubMed]
- Yue S, Mu W, Erb U, et al. The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding. Oncotarget 2015;6:2366-84. [Crossref] [PubMed]
- Kindt N, Journe F, Laurent G, et al. Involvement of macrophage migration inhibitory factor in cancer and novel therapeutic targets. Oncol Lett 2016;12:2247-53. [PubMed]
- Funamizu N, Hu C, Lacy C, et al. Macrophage migration inhibitory factor induces epithelial to mesenchymal transition, enhances tumor aggressiveness and predicts clinical outcome in resected pancreatic ductal adenocarcinoma. Int J Cancer 2013;132:785-94. [Crossref] [PubMed]
- Tomasek JJ, Gabbiani G, Hinz B, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3:349-63. [Crossref] [PubMed]
- Bobrie A, Krumeich S, Reyal F, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res 2012;72:4920-30. [Crossref] [PubMed]
- Wang Q, Ni Q, Wang X, et al. High expression of RAB27A and TP53 in pancreatic cancer predicts poor survival. Med Oncol 2015;32:372. [Crossref] [PubMed]
- Adamczyk KA, Klein-Scory S, Tehrani MM, et al. Characterization of soluble and exosomal forms of the EGFR released from pancreatic cancer cells. Life Sci 2011;89:304-12. [Crossref] [PubMed]
- Bloomston M, Bhardwaj A, Ellison EC, et al. Epidermal growth factor receptor expression in pancreatic carcinoma using tissue microarray technique. Dig Surg 2006;23:74-9. [Crossref] [PubMed]
- Ueda S, Ogata S, Tsuda H, et al. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas 2004;29:e1-8. [Crossref] [PubMed]
- Arscott WT, Camphausen KA. EGFR isoforms in exosomes as a novel method for biomarker discovery in pancreatic cancer. Biomark Med 2011;5:821. [Crossref] [PubMed]
- Klein-Scory S, Tehrani MM, Eilert-Micus C, et al. New insights in the composition of extracellular vesicles from pancreatic cancer cells: implications for biomarkers and functions. Proteome Sci 2014;12:50. [Crossref] [PubMed]
- Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654-9. [Crossref] [PubMed]
- Fabbri M, Paone A, Calore F, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A 2012;109:E2110-6. [Crossref] [PubMed]
- Rana S, Malinowska K, Zöller M. Exosomal Tumor MicroRNA Modulates Premetastatic Organ Cells. Neoplasia 2013;15:281-95. [Crossref] [PubMed]
- Dillhoff M, Wojcik SE, Bloomston M. MicroRNAs in Solid Tumors. J Surg Res 2009;154:349-54. [Crossref] [PubMed]
- Shen J, Stass SA, Jiang F. MicroRNAs as Potential Biomarkers in Human Solid Tumors. Cancer Lett 2013;329:125-36. [Crossref] [PubMed]
- Ali S, Dubaybo H, Brand RE, et al. Differential Expression of MicroRNAs in Tissues and Plasma Co-exists as a Biomarker for Pancreatic Cancer. J Cancer Sci Ther 2015;7:336-46. [Crossref] [PubMed]
- Madhavan B, Yue S, Galli U, et al. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int J Cancer 2015;136:2616-27. [Crossref] [PubMed]
- Gallo A, Tandon M, Alevizos I, et al. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One 2012;7:e30679 [Crossref] [PubMed]


