
The role of ethnicity in personalized dosing of small molecule tyrosine kinase inhibitors used in oncology
Introduction
The development of molecular targeted therapies, such as small molecule tyrosine kinase inhibitors (smTKIs), has revolutionized the treatment of some cancers, offering many patients a larger survival benefit with a lower toxicity profile and better quality of life, compared to traditional cytotoxic chemotherapy. However, despite their targeted mechanism of action, smTKIs exhibit large inter-individual variability in their systemic exposure (pharmacokinetics) and effects (pharmacodynamics) (1-3). Inter-individual variability in pharmacokinetics is particularly pertinent in oncology, as anticancer agents are frequently administered at doses close to maximally tolerable intensity, and most smTKIs are considered to have a narrow therapeutic index (3). Therefore, small changes in plasma concentrations may lead to serious adverse drug reactions or the potential for therapeutic failure (4). For some smTKIs, including axitinib, imatinib, sunitinib and pazopanib, there is sound evidence that systemic exposure correlates with clinical outcomes, thereby highlighting the importance of precision dosing (1,3,5). However, all smTKIs are still initiated at fixed doses, putting patients at risk of unpredictable efficacy and toxicity (3). Understanding reasons for variability in the pharmacokinetics and pharmacodynamics of smTKIs is fundamental to reducing the incidence of suboptimal outcomes.
There is growing evidence to suggest that ethnicity may be a factor contributing to the inter-individual variability observed in the exposure and response to smTKIs (6,7). The same dose of a smTKI prescribed to people from different ethnic backgrounds can result in different systemic exposures, efficacy and toxicity (8,9). Outcomes from smTKI treatment are influenced by a complex interplay of both intrinsic and extrinsic factors affecting their pharmacokinetic and pharmacodynamic pathways (6,10). Thus, inter-ethnic differences in the outcomes of treatment with smTKIs are likely a reflection of population differences in these intrinsic and extrinsic determinants (6). Intrinsic factors are those relating to an individual’s physiological characteristics, such as renal/hepatic function and body weight/composition, and genetic characteristics, considering both somatic and germline genetics (6,7). These include ethnic variation in the expression or activity of genes encoding for drug metabolizing enzymes and transporters (11-15), as well as genes involved in the mechanism of action of a drug, such as mutations in drug target proteins that cause particular sensitivities or resistance to the drug (15-19). Extrinsic factors, such as tobacco use, complementary and herbal medicine use and dietary habits, can vary between people of different geographic ancestries and can also influence the pharmacokinetics of a drug, its concentration at the target site, and thus drug response (6).
Recognition of inter-ethnic differences in anticancer treatment outcomes, as well as understanding reasons for these differences, can help identify populations that are predisposed to treatment resistance or susceptibility to adverse effects. The ethnicity of a patient could serve as a marker or predictor, alerting prescribers of patients who may need further investigation (e.g., genotyping critical pathways) to guide initial dose selection. It can also help identify patients who may need therapeutic drug monitoring to achieve adequate drug concentrations with minimal risk of harmful effects. Adverse drug reactions can greatly impact patient adherence, and may even result in cessation of treatment. Thus, identifying patients who are at risk of severe toxicities due to certain intrinsic/extrinsic factors, and personalizing their dose regimen with careful monitoring to reflect their pharmacokinetic and pharmacodynamic characteristics, can avoid treatment failure and improve a patients’ quality of life. By incorporating knowledge of a patients’ physiology, genetic predisposition and environmental influences into prescribing practices, we can move to a model of precision medicine and optimally utilize the life-changing drugs that are available. It may be possible to make earlier interventions, thereby reducing the likelihood of disease progression and ineffective treatment.
The aim of this review is to summarize the known information on inter-ethnic differences relevant for smTKIs in the treatment of cancer, discussing pharmacokinetic, efficacy and safety perspectives. This review will also illustrate how smTKI prescribing practices can be informed by considering a patients’ ethnicity.
Inter-ethnic differences in tyrosine kinase inhibitor treatment outcomes
The efficacy of some smTKIs has been found to differ between European and East Asian populations (Table 1). There is good evidence that people of East Asian ancestry have significantly higher response rates and superior survival outcomes to erlotinib, gefitinib, lapatinib and regorafenib, when compared to non-East Asian patients (8,9,44-48,51,65,66,83-85). No inter-ethnic differences in treatment response have been reported for ruxolitinib and osimertinib (71,72,86,87,114-116). Additionally, there are known ethnic differences in the tolerability profile of some smTKIs (Table 2). Many studies have demonstrated that Asian patients, in particular East Asians, experience more severe and more frequent smTKI-related adverse events when compared to people of European ancestry (79,90,97,121,125), with the exceptions of ceritinib and crizotinib, where Asian patients appear less susceptible to adverse events (32,118), and osimertinib, which does not appear to display inter-ethnic differences in harmful effects (71). Accordingly, with most smTKIs, higher rates of toxicity-related dose reductions, dose interruptions and drug discontinuations have been reported in patients of East Asian than European ancestry (20,45,46,51,52,67,76,89,124,125).
Full table
Full table
Inter-ethnic differences in pharmacokinetic pathways
Tyrosine kinase inhibitor pharmacokinetics
Pharmacokinetics describes the relationship between the drug dose and the resulting plasma and tissue drug concentrations (126). The processes of absorption, distribution, metabolism and excretion of a drug all contribute to the concentration-time profile observed in a patient undergoing smTKI treatment (4).
Absorption and bioavailability
For orally administered drugs, bioavailability influences systemic exposure (2), which is highly variable for most smTKIs (2). Bioavailability is a product of the fraction of the dose that is absorbed into enterocytes, the dose that reaches the hepatic portal vein unchanged, and the fraction that is not metabolized by enzymes in the liver (2,127). Therefore, membrane transporters and metabolizing enzymes can be important determinants of smTKI bioavailability (10,126,127). Once smTKIs enter the enterocyte from the gut lumen, they can undergo metabolism (10,126,127). Subsequently the smTKI may either undergo efflux back into the gut lumen, or be transferred into the portal circulation via passive or active (efflux) transport (10,126,127). Upon presentation to the liver via the portal circulation, smTKIs can be taken into the hepatocytes where they can be metabolized, excreted into bile or transported back to the systemic circulation (10,126,127).
Membrane transport proteins include ATP-dependent (ABC) and solute carrier transporters (SLC) (1,2). ABC transporters mediate drug efflux, and include P-glycoprotein (P-gp; encoded by ABCB1/MDR1), Breast Cancer Resistant Protein (BCRP; encoded by ABCG2) and Multidrug Resistance Protein (MRP; encoded by ABCC) (1,2,128,129). ABC transporters are expressed on various cells, including the apical/luminal membrane of enterocytes, where they can act as a barrier to intestinal drug absorption (2,128,129). All TKIs except for cabozantinib, ibrutinib, regorafenib, ruxolitinib and trametinib are substrates for either P-gp or BCRP efflux transporters (Table S1) (1,130). SLC transporters mediate drug uptake into the cell and include organic anion transporters OATP1B1 (SLC01B1) and OATP1B3 (SLC01B3), and the organic cation transporter OCT1 (SLC22A1) (1,2,131). SLC transporters are expressed on various cells, including the apical and basolateral membranes of enterocytes, where they facilitate intestinal absorption (1,2,131). They are also expressed on the basolateral membrane of hepatocytes, where they promote hepatocellular drug uptake required for substrate metabolism and biliary excretion (1,2,131). In vitro studies suggest that most smTKIs cannot be considered significant substrates for uptake transporters, with the exception of axitinib for OATP1B1 and OATP1B3, regorafenib for OATP1B1 as well as nintedanib and dasatinib for OCT1 (Table S1) (1).
Distribution
Many smTKIs have extensive tissue distribution, with an apparent volume of distribution typically between 100 and 1,000 L, and a terminal half-life between 24 to 48 hours (Table S1) (3,132). Additionally, all smTKIs are extensively protein bound (>90%) to albumin or the acute phase protein α1-acid glycoprotein (AGP) (3,132). Finally, membrane transporters play a key role in smTKI distribution (126). ABC transporters are expressed on capillary endothelial cells of tissue barriers (including the blood-brain barrier), acting as a barrier to penetration of substrate drugs (2,126,128,129). SmTKIs must also access cancer cells and intracellular molecular targets, in which case uptake into, and efflux out of, the target cell via transporters are key determinants of drug delivery and action (126). The ABC transporters may also contribute to multidrug resistance in tumors by removing substrates from cancer cells (133).
Metabolism
Drug metabolism usually occurs in the liver after distribution to the body, but some drugs undergo first-pass (pre-systemic) metabolism in the intestinal wall (enterocytes) and liver after oral administration prior to reaching the systemic circulation (134). SmTKIs are metabolized by phase 1 reactions, primarily catalyzed by cytochrome-P450 (CYP) enzymes, and phase 2 reactions catalyzed by enzymes including UDP-glucuronosyltransferases (UGTs) (Table S1) (134). Metabolism can render drug molecules inactive, or may produce a compound that is of equivalent or greater pharmacological activity (2). For example, sunitinib and imatinib are primarily metabolized by CYP3A4 to produce biologically active metabolites N-desethyl (SU12662) and N-demethylated piperizine, respectively, which have similar potencies to their parent drugs (135,136). Clinical responses must be considered in light of the concentrations of the parent drug and active metabolite. Sorafenib and erlotinib are also metabolized to pharmacologically active metabolites, sorafenib N-oxide and OSI-420, respectively (15,137). Since these metabolites are not present at high concentrations, they are not expected to play a major role in determining the clinical activity observed after administration of each parent compound (15,137).
Excretion
SmTKIs are primarily cleared through hepatic metabolism and P-gp mediated biliary excretion, with elimination of unchanged drug in urine accounting for less than 10% of total systemic clearance (Table S1) (134). Most smTKIs are extensively metabolized prior to biliary excretion, with the exception of afatinib, as it is mainly eliminated unchanged in feces (138). Membrane transporters play a role in the excretion of smTKIs. ABC transporters are expressed on bile caniculi of hepatocytes and are therefore involved in the biliary excretion of substrates. They are also expressed on renal epithelial cells, where they export substrates from the cytoplasm of the renal tubular cells to the urine (1,2,131).
Ethnic factors influencing pharmacokinetic determinants of TKIs
Expression levels and activities of drug transporters and metabolizing enzymes are influenced by genetic and environment factors, and may have important consequences for drug or metabolite(s) concentrations at the site of action and hence the efficacy and tolerability of smTKIs.
Genetic factors can be cis or trans acting elements (4). Cis acting elements include non-synonymous single nucleotide polymorphisms (SNPs), which are single nucleotide substitutions that lead to an amino acid change, a premature stop codon, or altered splicing (4). For example, the non-synonymous SNPs 34G>A (Val12Met) and 421C>A (Gln141Lys) in the coding regions (exon) of ABCG2 are associated with decreased BCRP expression and activity, reduced efflux and increased plasma and cellular exposure of several BCRP substrates (130,139-141). SNPs in introns (non-coding region) can also have functional consequences. The 6986A>G SNP in CYP3A5 (CYP3A5*3) creates an alternative splice acceptor site in intron 3, shifting the reading frame and causing a premature stop codon and non-functional protein (142). Conversely, the A allele is associated with the expresser phenotype of CYP3A5 (CYP3A5*1) (143). Synonymous SNPs can also affect the activity of the protein, and thus should not be disregarded (4,144). Although they do not result in an amino acid change, they can still cause “fitness consequences” and phenotypic differences (4,144). For example, the ABCB1 3435C>T SNP results in a synonymous change (ATC isoleucine, ATT isoleucine) that has been found to affect mRNA stability and the timing of co-translational folding, thereby altering P-gp conformation and the structure of interaction sites (144-146). However, its functional effect on P-gp expression is inconclusive, with studies associating the TT genotype with decreased P-gp expression (146-149), increased expression (150), or no effect (151). Haplotypes, which are SNPs that are inherited together in a particular pattern on the same chromatid, can also have pharmacokinetic implications (4). The ABCB1 3435T>C/1236T>C/2677T>G/A TTT haplotype combination results in decreased P-gp expression, and has been associated with increased plasma exposure of P-gp substrates (145). Furthermore, nucleotide insertions and deletions in exons and introns can affect protein structure and activity, by shifting the reading frame (4). Finally, sequence variations in the DNA binding site may affect the binding affinity of regulatory molecules (4). The presence of an additional TA repeat in the TATA sequence of the UGT1A1 promotor (UGT1A1*28) results in reduced transcription and reduced UGT1A1 enzyme activity (152). Trans acting elements can also contribute to pharmacokinetic variability. They include the nuclear receptors, PXR (pregnane X receptor, NR1I2) and CAR (constitutive androstane receptor, NR1I3), which regulate the transcription of genes encoding drug metabolizing enzymes and transporters (4,126). Polymorphisms in NR1I2 and NR1I3 can affect the expression and activity of metabolizing enzymes and transporters (153).
Inter-ethnic differences in the pharmacokinetics of a drug can be due to variability in allele frequencies, and in the types of allelic variants of drug metabolizing enzymes, transporters and nuclear receptors in people from different ethnic backgrounds (4,126). Many variants in drug metabolizing enzymes and transporters have been described, which can result in either loss-of-function of the protein or increased activity. An ethnic group with a higher prevalence of an allelic variant of an efflux transporter that has impaired function, would have reduced drug efflux, and therefore increased plasma and cellular concentrations of the drug at a standard dose (4). This could potentially result in more frequent and severe adverse drug reactions, compared to people from other ethnic populations who have a lower frequency of this allelic variant (4). Additionally, an ethnic population with a higher frequency of a metabolizing enzyme with increased activity, would have higher mean clearance and lower mean plasma exposure, and thus possibly reduced efficacy and/or less frequent adverse events (4). The clinical relevance of these polymorphisms depends on whether the drug of interest is a substrate of the metabolizing enzyme or transporter, whether plasma or tissue concentrations correspond to efficacy and toxicity, and whether the metabolites produced are pharmacologically active. Additionally, since smTKIs have a narrow therapeutic index, polymorphisms contributing to aberrant drug metabolism and transport can result in clinically significant changes to drug response (126).
Other environmental (non-genetic) factors may also contribute to ethnic variability in pharmacokinetics and therefore response to tyrosine kinase inhibitors, such as tobacco smoking, diet and the use of complementary and herbal medicines (4). These factors are discussed below.
Ethnic differences in genetic determinants of TKI pharmacokinetics
Many studies have correlated polymorphisms in genes encoding metabolizing enzymes or transporters with toxicity and efficacy of smTKIs. Variability in the frequency and the types of these genetic variants among people from different ethnic populations can, in part, explain the inter-ethnic differences observed in smTKI exposure, efficacy and adverse drug outcomes (Table 3).

Full table
Sunitinib
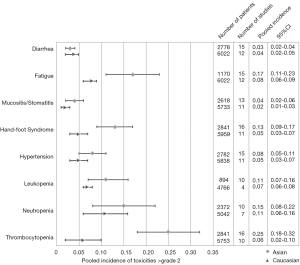
High sunitinib systemic exposure has been correlated with an increased risk of adverse events, as well as improved tumor response rates, time to progression (TTP) and overall survival (OS) in patients with gastrointestinal stromal tumors (GIST) and metastatic renal cell carcinoma (RCC) (209). A number of population pharmacokinetic studies have demonstrated that Asian patients have lower clearance of sunitinib and greater sunitinib plasma exposure compared to people from a non-Asian background (209-211). Accordingly, many studies have demonstrated that East Asians are more susceptible than people from a European background to sunitinib-related adverse events (Figure 1) (97,98,157,212-214).
This altered pharmacokinetic profile in East Asian patients can be explained in part by the higher prevalence of the ABCG2 421C>A allele and the ABCB1 3435CC genotype in East Asians than Europeans (154). Both variants are independently associated with significantly lower clearance and higher plasma exposure of sunitinib (143,158,159,169,171,215). The ABCG2 421AA genotype has also been correlated with a significantly higher risk of sunitinib-induced toxicities, including thrombocytopenia [odds ratio (OR) =9.90, P=0.04], neutropenia (OR =18.20, P=0.02) and Hand-Foot Syndrome (HFS) (OR =28.46, P=0.01) in Asian cohort studies (157-159). A case report in 2014 described a Japanese patient with RCC who was homozygous for ABCG2 421AA and developed severe thrombocytopenia, transaminase elevation, hypoxia, and pleural effusion on a 50 mg daily dose of sunitinib, due to elevated sunitinib and metabolite (SU12662) plasma concentrations (160). Additionally, Asian patients carrying the ABCB1 3435CC genotype had a significantly higher risk of all-grade rash [relative risk (RR) =3.00] and mucositis (RR =1.60), compared to T allele carriers (169). A separate study demonstrated a 10-fold reduction in the risk of neutropenia (P=0.01) and 3-fold reduction in the risk of diarrhoea (P=0.02) in patients expressing the ABCB1 3435T allele (171). Furthermore, the ABCB1 TTT haplotype (3435T>C/1236T>C/2677T>G/A), which is more prevalent in East Asians and South Asians than Europeans, has been associated with an increased risk of sunitinib-induced HFS (0R =2.56, P=0.035) (175). Recently, a case was reported of a Japanese patient that developed severe toxicities with sunitinib and gemcitabine, including grade 3 thrombocytopenia, neutropenia, respiratory distress, and elevated transaminases (173). The patient was found to have the ABCB1 TTT haplotype, as well as high sunitinib and SU12662 concentrations (173).
Metabolizing enzymes also play an important role in the inter-ethnic variability of sunitinib exposure and response. An exploratory study in patients with GIST and RCC found a significant correlation between the CYP1A1 2455A>G variant and an increased risk of leukopenia (OR =6.24, P=0.029) and mucosal inflammation (OR =4.03, P=0.021) (175). This G allelic variant is associated with increased CYP1A1 catalytic activity, and therefore is hypothesized to increase sunitinib conversion to the SU12662 metabolite (154). Excessive accumulation of SU12662 has been associated with grade 3 thrombocytopenia and leukopenia (216). Additionally, CYP3A5*1 has been associated with an increased risk of sunitinib dose reductions secondary to toxicity (180,181), and increased progression-free survival (PFS) in RCC patients treated with sunitinib [hazard ratio (HR) =0.266, P<0.05] (179). The increased catalytic activity of CYP3A5 associated with this variant results in increased conversion of sunitinib to SU12662 (143). It is hypothesized that this increased conversion to SU12662, which has a longer elimination half-life than sunitinib, results in increased exposure and therefore altered clinical outcomes (143). Furthermore, the CYP3A4 rs4646437G>A SNP has been correlated with an increased risk of hypertension in sunitinib treated RCC patients (OR =2.4, P=0.021) (177). It is hypothesized that hypertension is due to inhibition of vascular endothelial growth factor receptor-2 (VEGFR-2), leading to a reduced amount of nitric oxide and therefore vasoconstriction (177,217). All these CYP variants are more prevalent in South Asian and East Asian, compared to European populations (154), a possible explanation for the greater incidence of sunitinib-induced adverse events observed in Asians.
It has been suggested that people of Asian ancestry are started on lower doses of sunitinib to reduce the likelihood of severe toxicities (218). A study in Singapore evaluated sunitinib outcomes at an attenuated dosing regimen of 37.5 mg/day (4 weeks on, 2 weeks off), which is lower than the conventional dosing of 50 mg daily (4 weeks on, 2 weeks off) (218). Both regimens demonstrated comparable survival outcomes, however, there was a significantly lower rate of toxicities (P=0.0088) and toxicity-related dose reductions (P=0.005) with the attenuated dose regimen (218).
Imatinib
Higher imatinib systemic exposure is associated with improved response rates, time to response, and event-free survival in patients with chronic-myeloid leukaemia (CML) (161,219-222). In patients with GIST, imatinib systemic exposure is correlated to clinical response and disease progression (223). There is a growing body of evidence to suggest that East Asian patients with GIST or CML are more susceptible to imatinib-related adverse events, and are more likely to respond to treatment (39,60-63,224-228). This variability in response is possibly a reflection of inter-ethnic differences in imatinib exposure, due to inter-ethnic variability in the activity and expression of BCRP and P-gp.
A study conducted in Chinese patients with GIST correlated the T allele of ABCB1 3435T>C with significantly higher steady-state imatinib plasma concentrations (184). They hypothesized that this polymorphism resulted in reduced P-gp production, thereby lowering drug clearance (184). In a meta-analysis of ABCB1 gene polymorphisms, the T allele of ABCB1 3435T>C and G allele of ABCB1 2677T>G/A were predictors of worse imatinib response in patients with chronic-phase CML (170). Both of these allelic variants are more common in Europeans than East Asians, which could contribute to the observed inter-ethnic differences in imatinib outcomes (154). The ABCG2 421C>A allelic variant has also been correlated with decreased imatinib clearance, and increased imatinib plasma and cellular concentrations (141,161-163). A study in Korean patients with GIST demonstrated a significantly superior 5-year PFS rate in patients with the ABCG2 421AA genotype (92.3% vs. 65%, P=0.047) (164). Similarly, in CML patients, this AA genotype has been associated with increased major molecular response (MMR) rates (155). The ABCG2 34GG genotype, which is more prevalent in Europeans than East Asians, has also been correlated with significantly poorer cytogenetic response rates (major: MCyR, complete: CCyR) (155). This variant is associated with normal BCRP expression, thus reducing imatinib intestinal absorption and systemic exposure (229). Together, these studies indicate that genotyping for ABCG2 421C>A and 34G>A, as well as ABCB1 2677T>G/A and 3435T>C, may be useful in identifying patients at risk of sub-therapeutic response and toxicity, particularly among Asian populations. This may identify patients who will benefit from therapeutic drug monitoring, or dose-adjustment.
Variability in the activity of nuclear receptors can contribute to variability in treatment outcomes. A study in Chinese GIST patients correlated the CC wild-type genotype of NR1I2 rs3814055, with significantly higher imatinib trough plasma concentrations (P=0.0066) and a higher incidence of imatinib-induced edema (OR =13.48, P=0.003), compared to T allele carriers (184). The T allelic variant of rs3814055, which is more frequent in Europeans than East Asians (154), is associated with increased CYP3A4 and ABCB1 transcription and metabolic activity, and therefore increased imatinib clearance (153).
Erlotinib
There are known inter-ethnic differences in erlotinib pharmacokinetics and outcomes. East Asians are more susceptible than Europeans to erlotinib-related adverse events (45,46,121). Additionally, African Americans are reported to have higher erlotinib clearance and lower erlotinib systemic exposure, when compared to East Asians and people of European ancestry, as reflected in their substantially lower incidence of adverse events (120,230). It is known that erlotinib trough concentrations are an independent risk factor for the development of grade ≥2 diarrhea (P=0.037) and skin rash (P=0.031) (165), and therefore ethnic variability in erlotinib pharmacokinetic determinants has the potential to result in inter-ethnic differences in toxicity.
For example, the ABCB1 TTT haplotype (3435T>C/1236T>C/2677T>G/A) has been correlated with significantly higher erlotinib plasma exposure (P=0.021), and a greater risk of erlotinib-related grade 2/3 toxicities including skin rash (P=0.012) (171,174). Additionally, a population pharmacokinetic-pharmacodynamic model in Japanese patients noted a significantly higher incidence of grade ≥2 diarrhea in patients with the ABCG2 421C>A allelic variant (P=0.035) (165). This reduced function variant of BCRP is uncommon in African-Americans (154), and has been correlated with decreased erlotinib and OSI-420 clearance, higher erlotinib plasma exposure, and higher erlotinib cerebrospinal fluid penetration (165,231). Furthermore, a study in Japanese patients with non-small cell lung cancer (NSCLC) noted significantly higher erlotinib plasma exposure in patients carrying the CYP1A1 2455GG genotype (P=0.0151) and CYP3A5 6986GA/GG genotypes (P=0.0198) (176). These variants are more prevalent in East Asians than Europeans and African-Americans (154), a possible explanation for inter-ethnic differences in erlotinib adverse-event susceptibility. Conversely, the AA genotype of CYP3A5 (*1/*1) is found in 50% of African-Americans, compared to only 5.8% of East Asians and 4.5% of people of European ancestry (154).
Gefitinib
Inter-ethnic differences in BCRP expression may contribute to the ethnic disparities observed in gefitinib-related adverse events. The ABCG2 34G>A allelic variant has been correlated with gefitinib-induced skin toxicity (P=0.046) (156), and the ABCG2 421C>A allelic variant with grade ≥2 diarrhea (P=0.0046) (166,167). Both variants are associated with higher gefitinib plasma concentration at steady-state (166,167), an independent risk factor for developing gefitinib-induced diarrhea (P=0.006) and hepatotoxicity (P=0.024) (232). These variants are more prevalent in East Asians than Europeans (154), which is in line with the higher incidence of gefitinib-related adverse events observed in East Asians (51,52,121).
Sorafenib
East Asian patients have increased susceptibility to sorafenib-induced HFS (89-93,124,233-236). A study in Korean patients with hepatocellular carcinoma (HCC) identified the UGT1A9 IVS1-37431 AA variant as an independent risk factor for the development of high-grade HFS (OR =18.7, P=0.02) (183). Given that this genotype is more prevalent in East Asians than Europeans (154), it could explain some of the observed inter-ethnic variability in sorafenib toxicity (90).
Ethnic differences in physiological factors determining TKI pharmacokinetics
Plasma protein binding
Plasma protein binding has an important role in the distribution of anticancer drugs, including smTKIs (4,237-240). Many studies have demonstrated that a reduction in AGP concentration leads to an increase in the unbound fraction of imatinib and a reduction in total plasma imatinib concentrations, due to the rapid distribution of unbound imatinib into extravascular space and effects on hepatic clearance (238-240). Serum AGP concentrations have also been correlated with imatinib efficacy in CML, with a study suggesting that AGP concentrations (an inflammatory marker) are associated with the in vivo load of leukemic cells (240). There are inter-ethnic differences in plasma protein binding, with East Asian healthy subjects reported to have lower AGP concentrations than Europeans (241). A recent study in people with breast cancer noted significantly lower AGP concentrations in Chinese patients when compared to Malays and Indians (242). It could therefore be postulated that the greater toxicity and efficacy observed in East Asian patients treated with imatinib, could be a reflection of lower AGP concentrations, correlating to increased imatinib cellular and tissue distribution.
Body size and weight
For many drugs, body size (usually assessed by total body weight) can explain the inter-ethnic differences observed in pharmacokinetics. Generally, South Asian and East Asian populations have a higher portion of body fat with a lower body-mass-index (BMI), lean body mass (LBM), and body surface area (BSA), when compared to people of European ancestry (243-246). These factors have the potential to affect drug distribution and elimination. For lipophilic drugs, such as some smTKIs, apparent volume of distribution (V/F) can increase in people with a higher portion of body adipose tissue (247). For some drugs, lower LBM has been associated with lower clearance, related to organ size and blood flow (247). In population pharmacokinetic studies of imatinib, higher total body weight (TBW) has been correlated with increased V/F and clearance, consistent with increased body fat and body mass (248-250). Low TBW and BSA have also been correlated with higher imatinib plasma trough concentrations, and correspondingly improved response rates with increased toxicities (221,251). Additionally, low BMI and muscle mass have been identified as significant predictors of sorafenib (252) and sunitinib-related dose-limiting toxicities (253). Recently, LBM was also identified as a predictor of sunitinib plasma exposure and toxicities (215). Furthermore, lenvatinib clearance has been correlated to TBW (254). A subgroup analysis of patients enrolled in the SELECT trial demonstrated no difference in lenvatinib plasma exposure between the Japanese and non-Japanese subgroups after adjusting for TBW (67). For all aforementioned smTKIs, there is good evidence that East Asian patients are more susceptible than Europeans to drug-related adverse events (39,67,68,73,89,90,97,124) (Table 2). The lower LBM and higher body-fat percentage of East Asians could be a factor contributing to this inter-ethnic variability in tolerability. Therapeutic drug monitoring and body-weight based dosing may help reduce the incidence of severe smTKI-related adverse events, particularly in Asian patients.
Ethnic differences in extrinsic factors and TKI pharmacokinetics
Extrinsic factors should be considered as a possible source of inter-ethnic differences in drug response, as they can influence the pharmacokinetics and pharmacodynamics of smTKIs.
Complementary and herbal medicine use
The use of complementary and herbal medicines varies between people from different ethnic groups, due to different cultural and health beliefs, with the highest level of complementary and herbal medicine use reported in Asians (255,256). A study by Hsiao et al. noted greater use of green tea, ginseng, and soy products among Asian Americans compared to Americans of European ancestry (255). Additionally, complementary and herbal medicines are commonly used among cancer patients, with studies in Europe, America, Malaysia and Korea reporting use in 35.9%, 63%, 70.2% and 78.5% of patients, respectively (257-260). Importantly, many cancer patients are using complementary and herbal medicines in combination with their conventional anticancer therapy, with most patients (up to 72%) not informing their physicians about their complementary medicines (261). A study in patients undergoing treatment for melanoma demonstrated that 85.1% of patients that were using complementary and herbal medicine with their anticancer agent were at risk of drug interactions (262). Considering the narrow therapeutic index of most smTKIs, drug- drug interactions could lead to serious adverse events or reduced therapeutic effect of the smTKI.
Most smTKIs are metabolized primarily by CYP3A4, and are substrates of P-gp and BCRP membrane transporters (Table S1) (10,263). Therefore, there is a high potential for serious interactions when smTKIs are co-administered with complementary and herbal medicines that have modulatory effects on P-gp, BCRP and CYP3A4 (Figure 2) (10,263). Some herbal medicines that are inducers of CYP3A4, BCRP or P-gp have the potential to increase smTKI metabolism, and promote hepatic and renal excretion (10,263). The increased elimination and reduced plasma exposure of the smTKI could result in therapeutic failure. Conversely, inhibition of BCRP, P-gp or CYP3A4 by complementary and herbal medicines could result in enhanced intestinal absorption, reduced metabolism, and reduced renal and hepatic excretion (10,263). The increased smTKI exposure could result in severe drug-related toxicities. Case reports of these complementary medicine or herbal TKI interactions are presented in Table S2. Physicians must consider a patients’ use of complementary and herbal medicines prior to smTKI treatment, to ensure appropriate doses are initiated for efficacy and safety. Understanding inter-ethnic differences in the use of complementary and herbal medicines can also assist clinicians in educating patients, and in identifying possible reasons for suboptimal treatment outcomes.
Tobacco smoking
Tobacco smoking prevalence differs by ethnicity, both within and between countries. For example, in the US, the Center for Disease Control and Prevention reported that in 2010–2013 cigarette smoking prevalence was lowest in Asian Americans (10.9%) and highest in Native Americans (38.9%) (287). In addition, in many countries, including in Asia, smoking prevalence is higher in men than women. For example, in China in 2010 52.9% of men and 2.4% of women were reported to be current tobacco smokers (288).
Polycyclic aromatic hydrocarbons in tobacco smoke have the potential to affect drug metabolism, as they are potent CYP1A1 and CYP1A2 inducers (289). A pharmacokinetic study of erlotinib reported a 2.8-fold lower area under the concentration-time curve (AUC) in smokers and a 8.3-fold lower median steady-state plasma concentration (C24h) in smokers compared to non-smokers (290). Erlotinib is metabolized in part by CYP1A2 and CYP1A1, thereby resulting in greater erlotinib clearance and lower plasma concentrations in smokers. In a population pharmacokinetic analysis of NSCLC patients, the median C24h and clearance of erlotinib in current smokers were 60% and 143% of the values in a non-smoking group (291). Non-smokers also had a greater incidence of adverse events compared to smokers, consistent with higher erlotinib exposure (178). A meta-analysis of epidermal growth factor receptor (EGFR)-positive NSCLC patients treated with EGFR-smTKIs (erlotinib or gefitinib) demonstrated that non-smoking was associated with significantly prolonged PFS (HR=0.73, P=0.001) compared to ever smokers (292). The ethnic variability in cigarette smoking prevalence may be a factor contributing to superior response rates and greater toxicity observed in East Asian patients treated with these EGFR-smTKIs. Therefore, lower starting doses are recommended in heavy smokers (>20 cigarettes/day). A study in head and neck cancer patients’ demonstrated comparable efficacy outcomes in current smokers receiving an adjusted erlotinib dose of 300 mg daily, and in non-smokers receiving standard doses of 150 mg daily (293). With tobacco smoking potentially influencing drug response, population differences in the incidence of smoking can contribute to the inter-ethnic difference in efficacy and safety reported for some smTKIs.
Inter-ethnic differences in pharmacodynamic pathways
Pharmacodynamics refers to the relationship between drug concentrations and effects (126). Individuals with similar drug plasma or tissue concentrations can have significantly different responses, indicating that non-pharmacokinetic mechanisms can be involved in variable drug action (126). Pharmacodynamic variability can arise from genetic variability in the activity or expression of target genes or candidate genes involved in the therapeutic pharmacological pathway of a drug (10,126). The prevalence and activity of smTKI target variants in different ethnic populations can contribute to inter-ethnic differences in smTKI treatment outcomes.
Erlotinib and gefitinib
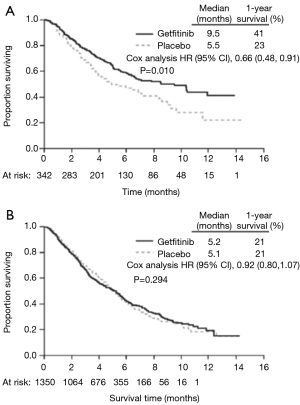
Many studies have demonstrated superior response rates and survival outcomes with erlotinib and gefitinib in East Asian patients compared to Europeans with NSCLC (Figure 3) (8,9,44-48,51-53,294-297). This variation in response has been correlated to inter-ethnic differences in the frequency of EGFR activating mutations, namely the in-frame deletion in exon 19 and L858R point mutation in exon 21 (201,202). These activating mutations are found in approximately 30% of East Asian patients with NSCLC, compared to only 8% of Europeans (201,202). EGFR-positive NSCLC patients show significantly greater tumor shrinkage, objective response rates (ORR), OS and PFS with gefitinib and erlotinib, compared to EGFR-negative patients (185-198), irrespective of the mutation type (197,199,200). However, studies selective for EGFR-positive NSCLC still show significantly superior outcomes with gefitinib and erlotinib in East Asians compared to other ethnic groups, indicating that other factors are also contributing to variable response (48). Similarly, East Asian patients with pancreatic cancer show more profound benefits with erlotinib than non-East Asians (49,50). This is likely due to inter-ethnic differences in EGFR mutation profiles, with EGFR activating mutations (L778P and I821T in exon 20, K728R and W731X in exon 19) significantly more common in Chinese than European patients with pancreatic cancer (50,203). These mutations are associated with significantly greater disease control rates (DCR), longer PFS and longer OS (50).
Imatinib
It appears that South Asian patients with CML do not respond as well as patients with an East Asian or European ancestry to imatinib treatment (224). Ethnic diversity in the expression of genes involved in imatinibs’ apoptotic pathway can explain some of the observed inter-ethnic variability in response. Bcl-2-like protein-1 (encoded by BCL2L11/BIM) plays a central role in the apoptosis of BCR-ABL cells, a pathway essential to imatinib-induced cell death (298). In a French cohort study, the T allelic variant of BCL2L11 465T>C significantly delayed MMR achievement (P=0.0407) and increased the risk of imatinib resistance (P=0.0049) (204). This variant is more common in South Asians than Europeans and East Asians (154), mirroring the poorer outcomes observed in South Asians. It is hypothesized that this polymorphism may reduce BIM activity and expression (204). Another candidate pathway involved in imatinib response is interferon (IFN) signaling. Variability in IFN-gamma expression affects hematopoietic stem cell expansion and proliferation, thereby altering the sensitivity of CML to imatinib (205). A study in CML patients correlated the CC genotype of IFNG rs2069705 with higher CCyR rates (HR =1.727, P=0.005) and MMR rates (HR =1.912, P=0.002) (205). Inter-ethnic differences in the frequency of the CC genotype correlate with the lower response rates observed in South Asian patients with chronic-phase CML (154).
Pazopanib
Pazopanib is an angiogenesis inhibitor targeting VEGFR, platelet-derived growth factor receptors (PDGFR) and the stem cell factor receptor, c-Kit. When pazopanib is used as second line treatment of advanced RCC, it appears that people of European ancestry have superior response rates and survival outcomes compared to East Asians (74,75). A polymorphism in the HIF1A gene (1790G>A) has been correlated with pazopanib efficacy, with the AG genotype associated with poorer PFS (20 vs. 44 weeks, P=0.03) and ORR (30% vs. 43%, P=0.02) compared to the GG wild-type (206). This variant results in higher transcriptional activity of the hypoxia-inducible factor-1 (HIF1) protein, which is a transcription factor that upregulates genes involved in angiogenesis, including vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF) (299,300). Therefore, patients with this variant have increased angiogenesis capability, rendering anti-angiogenesis agents like pazopanib less effective (299). The AG genotype is more common in East Asians than Europeans (154), which could potentially explain the inferior outcomes observed.
Sorafenib
East Asians are more susceptible than people with European ancestry to sorafenib-induced adverse events such as HFS and hypertension, and are therefore more likely to discontinue treatment (90). Inter-ethnic differences in genes relevant to tumor angiogenesis can explain some of the ethnic variability observed in sorafenib response. A cohort study in Korean patients with HCC identified the GG genotype of tumor-necrosis factor-α (TNF-α) -308G>A as an independent risk factor for developing high-grade sorafenib-induced HFS (OR =44, P=0.02) (183). This genotype is associated with higher levels of the TNF-α cytokine (183), resulting in increased anti-vascular and anti-angiogenic activity, and reduced tumor blood-flow (301,302). It is hypothesized that the poor vascular exchange associated with increased TNF-α, leads to an inflammatory response that manifests as HFS (183). This genotype is more prevalent in East Asians than Europeans (154), which may explain their enhanced susceptibility to sorafenib-induced HFS. In a small cohort study in Japanese patients with RCC, patients with the HLA-A*24 variant were at a significantly higher risk of sorafenib-induced HFS (207). Furthermore, a Korean case-series described three cases of sorafenib cutaneous reactions, of which two patients expressed HLA-A*24 (208). Binding of an antigenic drug to the human leukocyte antigen (HLA) protein activates cytotoxic T lymphocytes, a possible mechanism for skin toxicities (207). Interestingly, HLA-A*24 is more common in populations of Japanese ancestry than European ancestry (303), another explanation for the increased susceptibility to sorafenib-induced HFS in East Asians. However, larger studies are required to validate these associations.
Sunitinib
Genetic polymorphisms in sunitinib target proteins, such as VEGFR-2 and FMS-like tyrosine kinase-3 (FLT3), have been linked to increased sunitinib-induced toxicity (175). In an exploratory study of patients with GIST and RCC, the risk of sunitinib-induced high-grade toxicity was increased with the VEGFR-2 1191C>T allelic variant (OR =2.39, P=0.046) (175). This variant is associated with a lower binding efficiency of VEGF to the VEGFR-2 (304), and is more prevalent in East Asians than Europeans (154). This study also correlated leukopenia with the FLT3 738C>T allelic variant (OR =2.8, P=0.008) (175), which is also more prevalent in East Asians than Europeans (154). Similarly, a retrospective study of Asian RCC patients demonstrated a significantly increased risk of leukopenia (OR =8.0, P=0.03) and neutropenia (OR =2.7; P=0.04) in patients expressing the FLT3 738TT genotype (171). These studies suggest that Asian patients comprise a subgroup with increased potential for target-related adverse events when treated with sunitinib.
Challenges for clinical practice
Ethnicity is an important factor accounting for inter-individual differences in smTKI response. However, there are challenges faced by prescribers when translating this evidence into clinical practice. The concept of ethnicity is complex, and there is a lack of concordance across studies in the descriptions of ethnic groups. Some studies define patients as White vs. non-White, whilst others nominate nationality (e.g., Korean) or geographic ancestry. Another challenge faced is the limited sample size of many studies, which have insufficient patient numbers from each ethnic group to perform statistical analyses of potential inter-ethnic differences in treatment outcomes. Additionally, small sample sizes may allow potentially important but uncommon genetic polymorphisms to be missed. Furthermore, comprehensive datasets on smTKI outcomes, pharmacokinetic profiles and genetic variants in all ethnic groups are not available. Almost all research has described East Asian, European and African-American populations, and information on many other ethnic groups who utilize these treatments is not available. Moreover, there may be undefined factors which affect an individual’s response to treatment and which contribute to ethnic differences across populations. Understanding all of these factors is fundamental to precision medicine, in order to assist with drug and dose selection for a specific patient of a particular ancestry.
Conclusions
It is clear that ethnicity is an important factor accounting for some of the inter-individual differences observed in smTKI treatment outcomes. Identifying factors that influence outcomes of anticancer drugs, including smTKIs, is a crucial step toward enabling physicians to make personalized treatment decisions. Receiving an appropriate first-line treatment after a cancer diagnosis is critical, as early response has been shown to predict long-term PFS and OS (305-310). We know certain ethnic groups have altered expression/activity of metabolizing enzymes and transporters, thereby influencing smTKI pharmacokinetics and response. Additionally, some ethnic populations have a higher frequency of mutations in candidate genes or biological pathways associated with sensitivity to smTKIs, while others are more likely to have a higher frequency of mutations associated with smTKI resistance. Knowledge of these genetic polymorphisms involved in smTKI pharmacokinetic and pharmacodynamic pathways, and how they influence response, can enable personalized medicine using genotype-based drug and dose selection. Ethnicity could be used as a surrogate to identify patients at risk of severe toxicities or suboptimal treatment, triggering genotype testing. When considered in conjunction with non-genetic factors, such as body weight and extrinsic influences, ethnicity can be used to individualize therapy in terms of both initial drug and dose selection, and to identify patients who would benefit from therapeutic drug monitoring. Finally, understanding the influence of ethnicity on drug pharmacokinetics and pharmacodynamics will better inform the design of future targeted therapies, and also help improve the dose rationale for clinical trials of smTKIs.
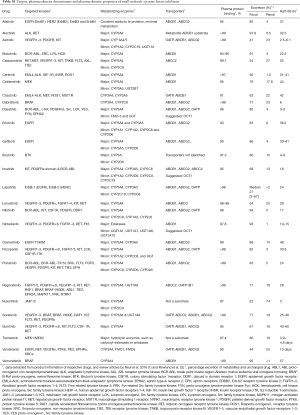
Full table
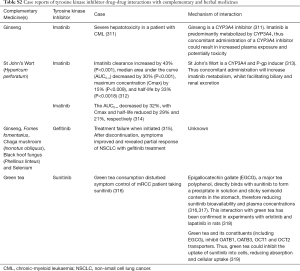
Full table
Acknowledgments
Funding: This work was supported by the Peter Coates Postgraduate Scholarship in Ethnopharmacology [to JA Touma].
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Michael Sorich and Andrew Rowland) for the series “Precision dosing of targeted anticancer drugs” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: AS Gross is an employee and shareholder of GSK. The others have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Neul C, Schaeffeler E, Sparreboom A, et al. Impact of membrane drug transporters on resistance to small-molecule tyrosine kinase inhibitors. Trends Pharmacol Sci 2016;37:904-32. [Crossref] [PubMed]
- Herbrink M, Nuijen B, Schellens JH, et al. Variability in bioavailability of small molecular tyrosine kinase inhibitors. Cancer Treat Rev 2015;41:412-22. [Crossref] [PubMed]
- Rowland A, van Dyk M, Mangoni AA, et al. Kinase inhibitor pharmacokinetics: comprehensive summary and roadmap for addressing inter-individual variability in exposure. Expert Opin Drug Metab Toxicol 2017;13:31-49. [Crossref] [PubMed]
- Syn NL, Yong WP, Lee SC, et al. Genetic factors affecting drug disposition in Asian cancer patients. Expert Opin Drug Metab Toxicol 2015;11:1879-92. [Crossref] [PubMed]
- de Wit D, Guchelaar HJ, den Hartigh J, et al. Individualized dosing of tyrosine kinase inhibitors:are we there yet? Drug Discov Today 2015;20:18-36. [Crossref] [PubMed]
- O'Donnell PH, Dolan ME. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res 2009;15:4806-14. [Crossref] [PubMed]
- Patel JN. Cancer pharmacogenomics: implications on ethnic diversity and drug response. Pharmacogenet Genomics 2015;25:223-30. [Crossref] [PubMed]
- Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) J Clin Oncol 2003;21:2237-46. [corrected]. [Crossref] [PubMed]
- Nishiwaki Y, Yano S, Tamura T, et al. Subset analysis of data in the Japanese patients with NSCLC from IDEAL 1 study on gefitinib. Gan To Kagaku Ryoho 2004;31:567-73. [PubMed]
- van Leeuwen RW, van Gelder T, Mathijssen RH, et al. Drug-drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol 2014;15:e315-26. [Crossref] [PubMed]
- Ozawa S, Soyama A, Saeki M, et al. Ethnic differences in genetic polymorphisms of CYP2D6, CYP2C19, CYP3As and MDR1/ABCB1. Drug Metab Pharmacokinet 2004;19:83-95. [Crossref] [PubMed]
- Liu JY, Qu K, Sferruzza AD, et al. Distribution of the UGT1A1*28 polymorphism in Caucasian and Asian populations in the US: a genomic analysis of 138 healthy individuals. Anticancer Drugs 2007;18:693-6. [Crossref] [PubMed]
- Kurose K, Sugiyama E, Saito Y. Population differences in major functional polymorphisms of pharmacokinetics/pharmacodynamics-related genes in Eastern Asians and Europeans: implications in the clinical trials for novel drug development. Drug Metab Pharmacokinet 2012;27:9-54. [Crossref] [PubMed]
- Myrand SP, Sekiguchi K, Man MZ, et al. Pharmacokinetics/genotype associations for major cytochrome P450 enzymes in native and first- and third-generation Japanese populations: comparison with Korean, Chinese, and Caucasian populations. Clin Pharmacol Ther 2008;84:347-61. [Crossref] [PubMed]
- Clark JW, Eder JP, Ryan D, et al. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res 2005;11:5472-80. [Crossref] [PubMed]
- Bell DW, Brannigan BW, Matsuo K, et al. Increased prevalence of EGFR-mutant lung cancer in women and in East Asian populations: analysis of estrogen-related polymorphisms. Clin Cancer Res 2008;14:4079-84. [Crossref] [PubMed]
- Weston MK, Moss DP, Stewart J, et al. Differences in breast cancer biological characteristics between ethnic groups in New Zealand. Breast Cancer Res Treat 2008;111:555-8. [Crossref] [PubMed]
- Chen P, Aldape K, Wiencke JK, et al. Ethnicity delineates different genetic pathways in malignant glioma. Cancer Res 2001;61:3949-54. [PubMed]
- Wiencke JK, Aldape K, McMillan A, et al. Molecular features of adult glioma associated with patient race/ethnicity, age, and a polymorphism in O6-methylguanine-DNA-methyltransferase. Cancer Epidemiol Biomarkers Prev 2005;14:1774-83. [Crossref] [PubMed]
- Kato T, Yoshioka H, Okamoto I, et al. Afatinib versus cisplatin plus pemetrexed in Japanese patients with advanced non-small cell lung cancer harboring activating EGFR mutations: Subgroup analysis of LUX-Lung 3. Cancer Sci 2015;106:1202-11. [Crossref] [PubMed]
- Wu YL, Sequist LV, Schuler M, et al. Overall survival with afatinib versus chemotherapy in patients with NSCLC harboring common EGFR mutations: subgroup analyses by race/ethnicity in LUX-Lung 3 and LUX-Lung 6. ESMO Asia 2015;445P.
- Gadgeel SM, Shaw AT, Govindan R, et al. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-small-cell lung cancer. J Clin Oncol 2016;34:4079-85. [Crossref] [PubMed]
- Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234-42. [Crossref] [PubMed]
- Hida T, Nakagawa K, Seto T, et al. Pharmacologic study (JP28927) of alectinib in Japanese patients with ALK+ non-small-cell lung cancer with or without prior crizotinib therapy. Cancer Sci 2016;107:1642-46. [Crossref] [PubMed]
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS):a randomised phase 3 trial. Lancet 2011;378:1931-39. [Crossref] [PubMed]
- Qin S, Bi F, Jin J, et al. Axitinib versus sorafenib as a second-line therapy in Asian patients with metastatic renal cell carcinoma: results from a randomized registrational study. Onco Targets Ther 2015;8:1363-73. [PubMed]
- Ueda T, Uemura H, Tomita Y, et al. Efficacy and safety of axitinib versus sorafenib in metastatic renal cell carcinoma: subgroup analysis of Japanese patients from the global randomized Phase 3 AXIS trial. Jpn J Clin Oncol 2013;43:616-28. [Crossref] [PubMed]
- Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood 2011;118:4567-76. [Crossref] [PubMed]
- Nakaseko C, Takahashi N, Ishizawa K, et al. A phase 1/2 study of bosutinib in Japanese adults with Philadelphia chromosome-positive chronic myeloid leukemia. Int J Hematol 2015;101:154-64. [Crossref] [PubMed]
- Elisei R, Schlumberger MJ, Muller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 2013;31:3639-46. [Crossref] [PubMed]
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR):final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016;17:917-27. [Crossref] [PubMed]
- Tan DS, Shaw AT, Mehra R, et al. Ceritinib in Asian versus Caucasian patients with advanced anaplastic lymphoma kinase (ALK)-rearranged (ALK+) NSCLC: Subgroup analysis of the ASCEND-1 trial. J Clin Oncol 2014;32:abstr 8078.
- Ascierto PA, McArthur GA, Dreno B, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 2016;17:1248-60. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Nishio M, Hirsh V, Kim DW, et al. Efficacy, safety, and patient-reported outcomes (PROs) with crizotinib versus chemotherapy in Asian patients in a phase III study of previously treated advanced ALK positive non-small cell lung cancer. J Thorac Oncol 2013;8:2818.
- Shaw AT, Solomon BJ. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2015;372:683-84. [Crossref] [PubMed]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65. [Crossref] [PubMed]
- Chuah CT, Nakamae H, Shen ZX, et al. Efficacy and safety of dasatinib versus imatinib in the East Asian subpopulation of the DASISION trial of newly diagnosed chronic myeloid leukemia in chronic phase. Leuk Lymphoma 2014;55:2093-100. [Crossref] [PubMed]
- Fujisawa S, Nakamae H, Ogura M, et al. Efficacy and safety of dasatinib versus imatinib in Japanese patients with newly diagnosed chronic-phase chronic myeloid leukemia (CML-CP):subset analysis of the DASISION trial with 2-year follow-up. Int J Hematol 2014;99:141-53. [Crossref] [PubMed]
- Nakamae H, Fujisawa S, Ogura M, et al. Dasatinib versus imatinib in Japanese patients with newly diagnosed chronic phase chronic myeloid leukemia: a subanalysis of the DASISION 5-year final report. Int J Hematol 2017;105:792-804. [Crossref] [PubMed]
- Sakamaki H, Ishizawa K, Taniwaki M, et al. Phase 1/2 clinical study of dasatinib in Japanese patients with chronic myeloid leukemia or Philadelphia chromosome-positive acute lymphoblastic leukemia. Int J Hematol 2009;89:332-41. [Crossref] [PubMed]
- Ottmann O, Dombret H, Martinelli G, et al. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood 2007;110:2309-15. [Crossref] [PubMed]
- Mok T, Wu YL, Au JS, et al. Efficacy and safety of erlotinib in 1242 East/South-East Asian patients with advanced non-small cell lung cancer. J Thorac Oncol 2010;5:1609-15. [Crossref] [PubMed]
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9. [Crossref] [PubMed]
- Wu YL, Kim JH, Park K, et al. Efficacy and safety of maintenance erlotinib in Asian patients with advanced non-small-cell lung cancer: a subanalysis of the phase III, randomized SATURN study. Lung Cancer 2012;77:339-45. [Crossref] [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [Crossref] [PubMed]
- Pilotto S, Di Maio M, Peretti U, et al. Predictors of outcome for patients with lung adenocarcinoma carrying the epidermal growth factor receptor mutation receiving 1st-line tyrosine kinase inhibitors: Sensitivity and meta-regression analysis of randomized trials. Crit Rev Oncol Hematol 2014;90:135-45. [Crossref] [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [Crossref] [PubMed]
- Wang JP, Wu CY, Yeh YC, et al. Erlotinib is effective in pancreatic cancer with epidermal growth factor receptor mutations: a randomized, open-label, prospective trial. Oncotarget 2015;6:18162-73. [Crossref] [PubMed]
- Chang A, Parikh P, Thongprasert S, et al. Gefitinib (IRESSA) in patients of Asian origin with refractory advanced non-small cell lung cancer: subset analysis from the ISEL study. J Thorac Oncol 2006;1:847-55. [Crossref] [PubMed]
- Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527-37. [Crossref] [PubMed]
- Guan ZZ, Zhang L, Li LY, et al. Efficacy of gefitinib on Chinese patients with locally advanced or metastatic non-small cell lung cancer: a clinical trial. Ai Zheng 2005;24:980-4. [PubMed]
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369:507-16. [Crossref] [PubMed]
- Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood 2015;126:739-45. [Crossref] [PubMed]
- Maruyama D, Nagai H, Fukuhara N, et al. Efficacy and safety of ibrutinib in Japanese patients with relapsed or refractory mantle cell lymphoma. Cancer Sci 2016;107:1785-90. [Crossref] [PubMed]
- Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010;362:2251-9. [Crossref] [PubMed]
- Nakamae H, Shibayama H, Kurokawa M, et al. Nilotinib as frontline therapy for patients with newly diagnosed Ph+ chronic myeloid leukemia in chronic phase: results from the Japanese subgroup of ENESTnd. Int J Hematol 2011;93:624-32. [Crossref] [PubMed]
- Wang J, Shen ZX, Saglio G, et al. Phase 3 study of nilotinib vs imatinib in Chinese patients with newly diagnosed chronic myeloid leukemia in chronic phase:ENESTchina. Blood 2015;125:2771-8. [Crossref] [PubMed]
- Nishida T, Shirao K, Sawaki A, et al. Efficacy and safety profile of imatinib mesylate (ST1571) in Japanese patients with advanced gastrointestinal stromal tumors: a phase II study (STI571B1202). Int J Clin Oncol 2008;13:244-51. [Crossref] [PubMed]
- Wan DS, Wu XJ, Pan ZZ, et al. Imatinib mesylate in the treatment of advanced gastrointestinal stromal tumors. Zhonghua Yi Xue Za Zhi 2006;86:3064-7. [PubMed]
- Ryu MH, Kang WK, Bang YJ, et al. A prospective, multicenter, phase 2 study of imatinib mesylate in korean patients with metastatic or unresectable gastrointestinal stromal tumor. Oncology 2009;76:326-32. [Crossref] [PubMed]
- Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004;364:1127-34. [Crossref] [PubMed]
- Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620-5. [Crossref] [PubMed]
- Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma:TRIO-013/LOGiC--A randomized phase III trial. J Clin Oncol 2016;34:443-51. [Crossref] [PubMed]
- Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014;32:2039-49. [Crossref] [PubMed]
- Kiyota N, Schlumberger M, Muro K, et al. Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci 2015;106:1714-21. [Crossref] [PubMed]
- Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621-30. [Crossref] [PubMed]
- Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 2015;16:1473-82. [Crossref] [PubMed]
- Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1):a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014;15:143-55. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643-52. [Crossref] [PubMed]
- Guo J, Jin J, Huang Y, et al. Comparison of PFS and safety for Asian compared to North American and European populations in the phase III trial of pazopanib versus sunitinib in patients with treatment-naive RCC (COMPARZ). J Clin Oncol 2013;31:366. [Crossref]
- Xie M, He CS, Huang JK, et al. Phase II study of pazopanib as second-line treatment after sunitinib in patients with metastatic renal cell carcinoma: a Southern China Urology Cancer Consortium Trial. Eur J Cancer 2015;51:595-603. [Crossref] [PubMed]
- Hainsworth JD, Rubin MS, Arrowsmith ER, et al. Pazopanib as second-line treatment after sunitinib or bevacizumab in patients with advanced renal cell carcinoma: a Sarah Cannon Oncology Research Consortium Phase II Trial. Clin Genitourin Cancer 2013;11:270-5. [Crossref] [PubMed]
- Kawai A, Araki N, Hiraga H, et al. A randomized, double-blind, placebo-controlled, Phase III study of pazopanib in patients with soft tissue sarcoma: results from the Japanese subgroup. Jpn J Clin Oncol 2016;46:248-53. [Crossref] [PubMed]
- Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med 2013;369:1783-96. [Crossref] [PubMed]
- Tojo A, Kyo T, Yamamoto K, et al. Ponatinib in Japanese patients with philadelphia chromosome-positive leukemia, a phase 1/2 study. Int J Hematol 2017;106:385-97. [Crossref] [PubMed]
- Yoshino T, Komatsu Y, Yamada Y, et al. Randomized phase III trial of regorafenib in metastatic colorectal cancer: analysis of the CORRECT Japanese and non-Japanese subpopulations. Invest New Drugs 2015;33:740-50. [Crossref] [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT):an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR):a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:619-29. [Crossref] [PubMed]
- Kim ST, Kim TW, Kim KP, et al. Regorafenib as Salvage Treatment in Korean Patients with Refractory Metastatic Colorectal Cancer. Cancer Res Treat 2015;47:790-5. [Crossref] [PubMed]
- Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID):an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302. [Crossref] [PubMed]
- Komatsu Y, Doi T, Sawaki A, et al. Regorafenib for advanced gastrointestinal stromal tumors following imatinib and sunitinib treatment: a subgroup analysis evaluating Japanese patients in the phase III GRID trial. Int J Clin Oncol 2015;20:905-12. [Crossref] [PubMed]
- Pavlakis N, Sjoquist KM, Martin AJ, et al. Regorafenib for the treatment of advanced gastric cancer (INTEGRATE):a multinational placebo-controlled phase II trial. J Clin Oncol 2016;34:2728-35. [Crossref] [PubMed]
- Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012;366:787-98. [Crossref] [PubMed]
- Jung CW, Shih LY, Xiao Z, et al. Efficacy and safety of ruxolitinib in Asian patients with myelofibrosis. Leuk Lymphoma 2015;56:2067-74. [Crossref] [PubMed]
- Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med 2015;372:426-35. [Crossref] [PubMed]
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125-34. [Crossref] [PubMed]
- Kudo M, Lencioni R, Marrero JA, et al. Regional differences in sorafenib-treated patients with hepatocellular carcinoma: GIDEON observational study. Liver Int 2016;36:1196-205. [Crossref] [PubMed]
- Kudo M, Ikeda M, Takayama T, et al. Safety and efficacy of sorafenib in Japanese patients with hepatocellular carcinoma in clinical practice: a subgroup analysis of GIDEON. J Gastroenterol 2016;51:1150-60. [Crossref] [PubMed]
- Ye SL, Chen X, Yang J, et al. Safety and efficacy of sorafenib therapy in patients with hepatocellular carcinoma: final outcome from the Chinese patient subset of the GIDEON study. Oncotarget 2016;7:6639-48. [Crossref] [PubMed]
- Kim DY, Kim HJ, Han KH, et al. Real-Life Experience of sorafenib treatment for hepatocellular carcinoma in Korea: from GIDEON Data. Cancer Res Treat 2016;48:1243-52. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Peixoto RD, Renouf DJ, Gill S, et al. Relationship of ethnicity and overall survival in patients treated with sorafenib for advanced hepatocellular carcinoma. J Gastrointest Oncol 2014;5:259-64. [PubMed]
- Liu X, Fiocco M, Swen JJ, et al. Assessment of ethnic differences in sunitinib outcome between Caucasian and Asian patients with metastatic renal cell carcinoma: a meta-analysis. Acta Oncol 2017;56:582-9. [Crossref] [PubMed]
- Lee SH, Bang YJ, Mainwaring P, et al. Sunitinib in metastatic renal cell carcinoma: an ethnic Asian subpopulation analysis for safety and efficacy. Asia Pac J Clin Oncol 2014;10:237-45. [Crossref] [PubMed]
- Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329-38. [Crossref] [PubMed]
- Shirao K, Nishida T, Doi T, et al. Phase I/II study of sunitinib malate in Japanese patients with gastrointestinal stromal tumor after failure of prior treatment with imatinib mesylate. Invest New Drugs 2010;28:866-75. [Crossref] [PubMed]
- Shen L, Qin S, Sun Y, et al. Sunitinib in Chinese patients with advanced gastrointestinal stromal tumor. J Clin Oncol 2015; [Epub ahead of print].
- Matsumoto K, Sawaki A, Mizuno N, et al. Clinical efficacy and safety of sunitinib after imatinib failure in Japanese patients with gastrointestinal stromal tumor. Jpn J Clin Oncol 2011;41:57-62. [Crossref] [PubMed]
- Li J, Gao J, Hong J, et al. Efficacy and safety of sunitinib in Chinese patients with imatinib-resistant or -intolerant gastrointestinal stromal tumors. Future Oncol 2012;8:617-24. [Crossref] [PubMed]
- Yoon DH, Ryu MH, Ryoo BY, et al. Sunitinib as a second-line therapy for advanced GISTs after failure of imatinib: relationship between efficacy and tumor genotype in Korean patients. Invest New Drugs 2012;30:819-27. [Crossref] [PubMed]
- Chen YY, Yeh CN, Cheng CT, et al. Sunitinib for Taiwanese patients with gastrointestinal stromal tumor after imatinib treatment failure or intolerance. World J Gastroenterol 2011;17:2113-9. [Crossref] [PubMed]
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107-14. [Crossref] [PubMed]
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30-9. [Crossref] [PubMed]
- Tsutsumida A, Yamazaki N, Takahashi A, et al. Evaluation of safety, tolerability, pharmacokinetics and efficacy of dabrafenib and trametinib combination therapy in Japanese patients with BRAF V600 mutation-positive advanced cutaneous melanoma:a phase I/II study. Ann Oncol 2015;26:ix103. [Crossref]
- Wells SA Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 2012;30:134-41. [Crossref] [PubMed]
- Uchino K, Komoda M, Tomomatsu J, et al. Safety and tolerability of vandetanib in Japanese patients with medullary thyroid cancer: a phase I/II open-label study. Endocr Pract 2017;23:149-56. [Crossref] [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [Crossref] [PubMed]
- McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3):extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014;15:323-32. [Crossref] [PubMed]
- Yamazaki N, Kiyohara Y, Sugaya N, et al. Phase I/II study of vemurafenib in patients with unresectable or recurrent melanoma with BRAF(V) (600) mutations. J Dermatol 2015;42:661-6. [Crossref] [PubMed]
- Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012;366:799-807. [Crossref] [PubMed]
- Oritani K, Okamoto S, Tauchi T, et al. A multinational, open-label, phase 2 study of ruxolitinib in Asian patients with myelofibrosis: Japanese subset analysis. Int J Hematol 2015;101:295-304. [Crossref] [PubMed]
- Du X, Zhou D. Efficacy and safety of JAK inhibitor INC424 in patients with primary and post-polycythemia vera or post-essential thrombocythemia myelofibrosis in the Chinese population. Front Med 2016;10:437-43. [Crossref] [PubMed]
- Hsyu PH, Gogat K, Duvillie L, et al. Pharmacokinetics and tolerability of bosutinib in Asian versus Non-Asian patients with philadelphia chromosome–positive leukemia. Blood 2012;120:4440.
- Hida T, Shi Y, Ahn MJ, et al. Exploratory subgroup analysis of crizotinib efficacy and safety in Asian and non-Asian patients with advanced ALK-positive non-small cell lung cancer (NSCLC) enrolled in a global phase II study. J Thorac Oncol 2012;7:5. [PubMed]
- Ou SH, Tang Y, Polli A, et al. Characterization of heart rate (HR) changes during crizotinib treatment:A retrospective analysis of 1,053 ALK+ NSCLC patients. J Clin Oncol 2014;32:8106.
- Phelps MA, Stinchcombe TE, Blachly JS, et al. Erlotinib in African Americans with advanced non-small cell lung cancer: a prospective randomized study with genetic and pharmacokinetic analyses. Clin Pharmacol Ther 2014;96:182-91. [Crossref] [PubMed]
- Shi L, Tang J, Tong L, et al. Risk of interstitial lung disease with gefitinib and erlotinib in advanced non-small cell lung cancer: a systematic review and meta-analysis of clinical trials. Lung Cancer 2014;83:231-9. [Crossref] [PubMed]
- Okamoto I, Kaneda H, Satoh T, et al. Phase I safety, pharmacokinetic, and biomarker study of BIBF 1120, an oral triple tyrosine kinase inhibitor in patients with advanced solid tumors. Mol Cancer Ther 2010;9:2825-33. [Crossref] [PubMed]
- Mross K, Stefanic M, Gmehling D, et al. Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clin Cancer Res 2010;16:311-9. [Crossref] [PubMed]
- Naito S, Tsukamoto T, Murai M, et al. Overall survival and good tolerability of long-term use of sorafenib after cytokine treatment: final results of a phase II trial of sorafenib in Japanese patients with metastatic renal cell carcinoma. BJU Int 2011;108:1813-9. [Crossref] [PubMed]
- Sorich MJ, Rowland A, Kichenadasse G, et al. Risk factors of proteinuria in renal cell carcinoma patients treated with VEGF inhibitors: a secondary analysis of pooled clinical trial data. Br J Cancer 2016;114:1313-7. [Crossref] [PubMed]
- Roden DM, George AL Jr. The genetic basis of variability in drug responses. Nat Rev Drug Discov 2002;1:37-44. [Crossref] [PubMed]
- Shugarts S, Benet LZ. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm Res 2009;26:2039-54. [Crossref] [PubMed]
- Zhou SF. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica 2008;38:802-32. [Crossref] [PubMed]
- Köck K, Brouwer KL. A perspective on efflux transport proteins in the liver. Clin Pharmacol Ther 2012;92:599-612. [Crossref] [PubMed]
- Burger H, van Tol H, Boersma AW, et al. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood 2004;104:2940-2. [Crossref] [PubMed]
- Giacomini KM, Huang SM, Tweedie DJ, et al. Membrane transporters in drug development. Nat Rev Drug Discov 2010;9:215-36. [Crossref] [PubMed]
- Scheffler M, Di Gion P, Doroshyenko O, et al. Clinical pharmacokinetics of tyrosine kinase inhibitors: Focus on 4-anilinoquinazolines. Clin Pharmacokinet 2011;50:371-403. [Crossref] [PubMed]
- Beuselinck B, Karadimou A, Lambrechts D, et al. Single-nucleotide polymorphisms associated with outcome in metastatic renal cell carcinoma treated with sunitinib. Br J Cancer 2013;108:887-900. [Crossref] [PubMed]
- van Erp NP, Gelderblom H, Guchelaar HJ. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev 2009;35:692-706. [Crossref] [PubMed]
- Goodman VL, Rock EP, Dagher R, et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res 2007;13:1367-73. [Crossref] [PubMed]
- Gschwind HP, Pfaar U, Waldmeier F, et al. Metabolism and disposition of imatinib mesylate in healthy volunteers. Drug Metab Dispos 2005;33:1503-12. [Crossref] [PubMed]
- Ling J, Johnson KA, Miao Z, et al. Metabolism and excretion of erlotinib, a small molecule inhibitor of epidermal growth factor receptor tyrosine kinase, in healthy male volunteers. Drug Metab Dispos 2006;34:420-6. [PubMed]
- Stopfer P, Marzin K, Narjes H, et al. Afatinib pharmacokinetics and metabolism after oral administration to healthy male volunteers. Cancer Chemother Pharmacol 2012;69:1051-61. [Crossref] [PubMed]
- Furukawa T, Wakabayashi K, Tamura A, et al. Major SNP (Q141K) variant of human ABC transporter ABCG2 undergoes lysosomal and proteasomal degradations. Pharm Res 2009;26:469-79. [Crossref] [PubMed]
- Noguchi K, Katayama K, Sugimoto Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: basic and clinical perspectives for molecular cancer therapeutics. Pharmgenomics Pers Med 2014;7:53-64. [Crossref] [PubMed]
- Nakanishi T, Ross DD. Breast cancer resistance protein (BCRP/ABCG2):its role in multidrug resistance and regulation of its gene expression. Chin J Cancer 2012;31:73-99. [Crossref] [PubMed]
- Kuehl P, Zhang J, Lin Y, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet 2001;27:383-91. [Crossref] [PubMed]
- Diekstra MH, Klumpen HJ, Lolkema MP, et al. Association analysis of genetic polymorphisms in genes related to sunitinib pharmacokinetics, specifically clearance of sunitinib and SU12662. Clin Pharmacol Ther 2014;96:81-9. [Crossref] [PubMed]
- Sauna ZE, Kimchi-Sarfaty C, Ambudkar SV, et al. Silent polymorphisms speak: how they affect pharmacogenomics and the treatment of cancer. Cancer Res 2007;67:9609-12. [Crossref] [PubMed]
- Kimchi-Sarfaty C, Oh JM, Kim IW, et al. A "silent" polymorphism in the MDR1 gene changes substrate specificity. Science 2007;315:525-8. [Crossref] [PubMed]
- Hoffmeyer S, Burk O, von Richter O, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA 2000;97:3473-8. [Crossref] [PubMed]
- Hitzl M, Drescher S, van der Kuip H, et al. The C3435T mutation in the human MDR1 gene is associated with altered efflux of the P-glycoprotein substrate rhodamine 123 from CD56+ natural killer cells. Pharmacogenetics 2001;11:293-8. [Crossref] [PubMed]
- Sauer G, Kafka A, Grundmann R, et al. Basal expression of the multidrug resistance gene 1 (MDR-1) is associated with the TT genotype at the polymorphic site C3435T in mammary and ovarian carcinoma cell lines. Cancer Lett 2002;185:79-85. [Crossref] [PubMed]
- Takane H, Kobayashi D, Hirota T, et al. Haplotype-oriented genetic analysis and functional assessment of promoter variants in the MDR1 (ABCB1) gene. J Pharmacol Exp Ther 2004;311:1179-87. [Crossref] [PubMed]
- Dey S. Single nucleotide polymorphisms in human P-glycoprotein: its impact on drug delivery and disposition. Expert Opin Drug Deliv 2006;3:23-35. [Crossref] [PubMed]
- Owen A, Goldring C, Morgan P, et al. Relationship between the C3435T and G2677T(A) polymorphisms in the ABCB1 gene and P-glycoprotein expression in human liver. Br J Clin Pharmacol 2005;59:365-70. [Crossref] [PubMed]
- Iyer L, Das S, Janisch L, et al. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J 2002;2:43-7. [Crossref] [PubMed]
- Lamba J, Lamba V, Schuetz E. Genetic variants of PXR (NR1I2) and CAR (NR1I3) and their implications in drug metabolism and pharmacogenetics. Curr Drug Metab 2005;6:369-83. [Crossref] [PubMed]
- Sherry ST, Ward M, Kholodov M, et al. dbSNP—database for single nucleotide polymorphisms and other classes of minor genetic variation. Genome Res 1999;9:677-9. [PubMed]
- Kim DH, Sriharsha L, Xu W, et al. Clinical relevance of a pharmacogenetic approach using multiple candidate genes to predict response and resistance to imatinib therapy in chronic myeloid leukemia. Clin Cancer Res 2009;15:4750-8. [Crossref] [PubMed]
- Tamura M, Kondo M, Horio M, et al. Genetic polymorphisms of the adenosine triphosphate-binding cassette transporters (ABCG2, ABCB1) and gefitinib toxicity. Nagoya J Med Sci 2012;74:133-40. [PubMed]
- Kim HR, Park HS, Kwon WS, et al. Pharmacogenetic determinants associated with sunitinib-induced toxicity and ethnic difference in Korean metastatic renal cell carcinoma patients. Cancer Chemother Pharmacol 2013;72:825-35. [Crossref] [PubMed]
- Mizuno T, Fukudo M, Fukuda T, et al. The effect of ABCG2 genotype on the population pharmacokinetics of sunitinib in patients with renal cell carcinoma. Ther Drug Monit 2014;36:310-6. [Crossref] [PubMed]
- Mizuno T, Fukudo M, Terada T, et al. Impact of genetic variation in breast cancer resistance protein (BCRP/ABCG2) on sunitinib pharmacokinetics. Drug Metab Pharmacokinet 2012;27:631-9. [Crossref] [PubMed]
- Miura Y, Imamura CK, Fukunaga K, et al. Sunitinib-induced severe toxicities in a Japanese patient with the ABCG2 421 AA genotype. BMC Cancer 2014;14:964. [Crossref] [PubMed]
- Takahashi N, Miura M, Scott SA, et al. Influence of CYP3A5 and drug transporter polymorphisms on imatinib trough concentration and clinical response among patients with chronic phase chronic myeloid leukemia. J Hum Genet 2010;55:731-7. [Crossref] [PubMed]
- Petain A, Kattygnarath D, Azard J, et al. Population pharmacokinetics and pharmacogenetics of imatinib in children and adults. Clin Cancer Res 2008;14:7102-9. [Crossref] [PubMed]
- Imai Y, Nakane M, Kage K, et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther 2002;1:611-6. [PubMed]
- Koo DH, Ryu MH, Ryoo BY, et al. Association of ABCG2 polymorphism with clinical efficacy of imatinib in patients with gastrointestinal stromal tumor. Cancer Chemother Pharmacol 2015;75:173-82. [Crossref] [PubMed]
- Fukudo M, Ikemi Y, Togashi Y, et al. Population pharmacokinetics/pharmacodynamics of erlotinib and pharmacogenomic analysis of plasma and cerebrospinal fluid drug concentrations in Japanese patients with non-small cell lung cancer. Clin Pharmacokinet 2013;52:593-609. [Crossref] [PubMed]
- Cusatis G, Gregorc V, Li J, et al. Pharmacogenetics of ABCG2 and adverse reactions to gefitinib. J Natl Cancer Inst 2006;98:1739-42. [Crossref] [PubMed]
- Li J, Cusatis G, Brahmer J, et al. Association of variant ABCG2 and the pharmacokinetics of epidermal growth factor receptor tyrosine kinase inhibitors in cancer patients. Cancer Biol Ther 2007;6:432-8. [Crossref] [PubMed]
- Hodges LM, Markova SM, Chinn LW, et al. Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein). Pharmacogenet Genomics 2011;21:152-61. [Crossref] [PubMed]
- Teo YL, Wee HL, Chue XP, et al. Effect of the CYP3A5 and ABCB1 genotype on exposure, clinical response and manifestation of toxicities from sunitinib in Asian patients. Pharmacogenomics J 2016;16:47-53. [Crossref] [PubMed]
- Zheng Q, Wu H, Yu Q, et al. ABCB1 polymorphisms predict imatinib response in chronic myeloid leukemia patients: a systematic review and meta-analysis. Pharmacogenomics J 2015;15:127-34. [Crossref] [PubMed]
- Chu YH, Li H, Tan HS, et al. Association of ABCB1 and FLT3 polymorphisms with toxicities and survival in asian patients receiving sunitinib for renal cell carcinoma. PLoS One 2015;10:e0134102 [Crossref] [PubMed]
- Fung KL, Gottesman MM. A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim Biophys Acta 2009;1794:860-71. [Crossref] [PubMed]
- Takayoshi K, Sagara K, Uchino K, et al. A case of metastatic renal cell carcinoma and bile duct carcinoma treated with a combination of sunitinib and gemcitabine. BMC Cancer 2015;15:426. [Crossref] [PubMed]
- Hamada A, Sasaki J, Saeki S, et al. Association of ABCB1 polymorphisms with erlotinib pharmacokinetics and toxicity in Japanese patients with non-small-cell lung cancer. Pharmacogenomics 2012;13:615-24. [Crossref] [PubMed]
- van Erp NP, Eechoute K, van der Veldt AA, et al. Pharmacogenetic pathway analysis for determination of sunitinib-induced toxicity. J Clin Oncol 2009;27:4406-12. [Crossref] [PubMed]
- Kai Y, Hamada A, Sasaki Ji, et al. Association of CYP1A1 and CYP3A5 polymorphisms with pharmacokinetics of erlotinib in patients with non-small cell lung cancer. Cancer Res 2011;71:Suppl 5459.
- Diekstra MH, Belaustegui A, Swen JJ, et al. Sunitinib-induced hypertension in CYP3A4 rs4646437 A-allele carriers with metastatic renal cell carcinoma. Pharmacogenomics J 2017;17:42-6. [Crossref] [PubMed]
- Lamba J, Hebert JM, Schuetz EG, et al. PharmGKB summary: very important pharmacogene information for CYP3A5. Pharmacogenet Genomics 2012;22:555-8. [Crossref] [PubMed]
- van der Veldt AA, Eechoute K, Gelderblom H, et al. Genetic polymorphisms associated with a prolonged progression-free survival in patients with metastatic renal cell cancer treated with sunitinib. Clin Cancer Res 2011;17:620-9. [Crossref] [PubMed]
- Diekstra MH, Swen JJ, Boven E, et al. CYP3A5 and ABCB1 polymorphisms as predictors for sunitinib outcome in metastatic renal cell carcinoma. Eur Urol 2015;68:621-9. [Crossref] [PubMed]
- Garcia-Donas J, Esteban E, Leandro-Garcia LJ, et al. Single nucleotide polymorphism associations with response and toxic effects in patients with advanced renal-cell carcinoma treated with first-line sunitinib:a multicentre, observational, prospective study. Lancet Oncol 2011;12:1143-50. [Crossref] [PubMed]
- Barbarino JM, Haidar CE, Klein TE, et al. PharmGKB summary: very important pharmacogene information for UGT1A1. Pharmacogenet Genomics 2014;24:177-83. [Crossref] [PubMed]
- Lee JH, Chung YH, Kim JA, et al. Genetic predisposition of hand-foot skin reaction after sorafenib therapy in patients with hepatocellular carcinoma. Cancer 2013;119:136-42. [Crossref] [PubMed]
- Liu J, Chen Z, Chen H, et al. Genetic polymorphisms contribute to the individual variations of imatinib mesylate plasma levels and adverse reactions in Chinese GIST patients. Int J Mol Sci 2017;18:603. [Crossref] [PubMed]
- Zbuk KM, Eng C. Predicting response to EGFR-tyrosine kinase inhibitors among diverse ancestries: just way too polymorphic. Cancer Biol Ther 2007;6:112-5. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11. [Crossref] [PubMed]
- Gazdar AF. Personalized medicine and inhibition of EGFR signaling in lung cancer. N Engl J Med 2009;361:1018-20. [Crossref] [PubMed]
- Hirsch FR, Varella-Garcia M, Bunn PA Jr, et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol 2006;24:5034-42. [Crossref] [PubMed]
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Ichihara S, Toyooka S, Fujiwara Y, et al. The impact of epidermal growth factor receptor gene status on gefitinib-treated Japanese patients with non-small-cell lung cancer. Int J Cancer 2007;120:1239-47. [Crossref] [PubMed]
- Han SW, Kim TY, Hwang PG, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol 2005;23:2493-501. [Crossref] [PubMed]
- Brugger W, Triller N, Blasinska-Morawiec M, et al. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. J Clin Oncol 2011;29:4113-20. [Crossref] [PubMed]
- Yang CH, Yu CJ, Shih JY, et al. Specific EGFR mutations predict treatment outcome of stage IIIB/IV patients with chemotherapy-naive non-small-cell lung cancer receiving first-line gefitinib monotherapy. J Clin Oncol 2008;26:2745-53. [Crossref] [PubMed]
- Kimura H, Kasahara K, Kawaishi M, et al. Detection of epidermal growth factor receptor mutations in serum as a predictor of the response to gefitinib in patients with non-small-cell lung cancer. Clin Cancer Res 2006;12:3915-21. [Crossref] [PubMed]
- Maruyama R, Nishiwaki Y, Tamura T, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 2008;26:4244-52. [Crossref] [PubMed]
- Ahn MJ, Park BB, Ahn JS, et al. Are there any ethnic differences in molecular predictors of erlotinib efficacy in advanced non-small cell lung cancer? Clin Cancer Res 2008;14:3860-6. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Asahina H, Yamazaki K, Kinoshita I, et al. A phase II trial of gefitinib as first-line therapy for advanced non-small cell lung cancer with epidermal growth factor receptor mutations. Br J Cancer 2006;95:998-1004. [Crossref] [PubMed]
- Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol 2008;26:2442-9. [Crossref] [PubMed]
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. [Crossref] [PubMed]
- Steuer CE, Behera M, Berry L, et al. Role of race in oncogenic driver prevalence and outcomes in lung adenocarcinoma: Results from the Lung Cancer Mutation Consortium. Cancer 2016;122:766-72. [Crossref] [PubMed]
- Tzeng CW, Frolov A, Frolova N, et al. Epidermal growth factor receptor (EGFR) is highly conserved in pancreatic cancer. Surgery 2007;141:464-9. [Crossref] [PubMed]
- Augis V, Airiau K, Josselin M, et al. A single nucleotide polymorphism in cBIM is associated with a slower achievement of major molecular response in chronic myeloid leukaemia treated with imatinib. PLoS One 2013;8:e78582 [Crossref] [PubMed]
- Kim DH, Kong JH, Byeun JY, et al. The IFNG (IFN-gamma) genotype predicts cytogenetic and molecular response to imatinib therapy in chronic myeloid leukemia. Clin Cancer Res 2010;16:5339-50. [Crossref] [PubMed]
- Xu CF, Bing NX, Ball HA, et al. Pazopanib efficacy in renal cell carcinoma: evidence for predictive genetic markers in angiogenesis-related and exposure-related genes. J Clin Oncol 2011;29:2557-64. [Crossref] [PubMed]
- Tsuchiya N, Narita S, Inoue T, et al. Risk factors for sorafenib-induced high-grade skin rash in Japanese patients with advanced renal cell carcinoma. Anticancer Drugs 2013;24:310-4. [Crossref] [PubMed]
- Sohn KH, Oh SY, Lim KW, et al. Sorafenib induces delayed-onset cutaneous hypersensitivity:a case series. Allergy Asthma Immunol Res 2015;7:304-7. [Crossref] [PubMed]
- Houk BE, Bello CL, Poland B, et al. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol 2010;66:357-71. [Crossref] [PubMed]
- Houk BE, Bello CL, Kang D, et al. A population pharmacokinetic meta-analysis of sunitinib malate (SU11248) and its primary metabolite (SU12662) in healthy volunteers and oncology patients. Clin Cancer Res 2009;15:2497-506. [Crossref] [PubMed]
- Khosravan R, Motzer RJ, Fumagalli E, et al. Population Pharmacokinetic/Pharmacodynamic Modeling of Sunitinib by Dosing Schedule in Patients with Advanced Renal Cell Carcinoma or Gastrointestinal Stromal Tumor. Clin Pharmacokinet 2016;55:1251-69. [Crossref] [PubMed]
- Li XS, Song Y, Gong K, et al. Clinical study of Sunitinib in the treatment of metastatic renal clear cell carcinoma: a single center 23 cases experience. Zhonghua Wai Ke Za Zhi 2010;48:375-7. [PubMed]
- Tomita Y, Shinohara N, Yuasa T, et al. Overall survival and updated results from a phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma. Jpn J Clin Oncol 2010;40:1166-72. [Crossref] [PubMed]
- Kim HS, Hong MH, Kim K, et al. Sunitinib for Asian patients with advanced renal cell carcinoma: a comparable efficacy with different toxicity profiles. Oncology 2011;80:395-405. [Crossref] [PubMed]
- Narjoz C, Cessot A, Thomas-Schoemann A, et al. Role of the lean body mass and of pharmacogenetic variants on the pharmacokinetics and pharmacodynamics of sunitinib in cancer patients. Invest New Drugs 2015;33:257-68. [Crossref] [PubMed]
- Takasaki S, Kikuchi M, Kawasaki Y, et al. Severe toxicity induced by accumulation of active sunitinib metabolite in a Japanese patient with renal cell carcinoma: a case report. J Med Case Rep 2017;11:28. [Crossref] [PubMed]
- Eechoute K, van der Veldt AA, Oosting S, et al. Polymorphisms in endothelial nitric oxide synthase (eNOS) and vascular endothelial growth factor (VEGF) predict sunitinib-induced hypertension. Clin Pharmacol Ther 2012;92:503-10. [PubMed]
- Tan HS, Li H, Hong YW, et al. Efficacy and safety of an attenuated-dose sunitinib regimen in metastatic renal cell carcinoma: results from a prospective registry in singapore. Clin Genitourin Cancer 2015;13:e285-95. [Crossref] [PubMed]
- Takahashi N, Wakita H, Miura M, et al. Correlation between imatinib pharmacokinetics and clinical response in Japanese patients with chronic-phase chronic myeloid leukemia. Clin Pharmacol Ther 2010;88:809-13. [Crossref] [PubMed]
- Picard S, Titier K, Etienne G, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood 2007;109:3496-9. [Crossref] [PubMed]
- Larson RA, Druker BJ, Guilhot F, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood 2008;111:4022-8. [Crossref] [PubMed]
- Guilhot F, Hughes TP, Cortes J, et al. Imatinib (IM) pharmacokinetic (PK) exposure and its correlation with clinical outcome in patients with chronic-phase chronic myeloid leukemia (CML-CP) for 400 Mg and 800 mg daily doses (Tyrosine Kinase Dose Optimization Study [TOPS]). Blood 2008;112:447. [PubMed]
- von Mehren M, Widmer N. Correlations between imatinib pharmacokinetics, pharmacodynamics, adherence, and clinical response in advanced metastatic gastrointestinal stromal tumor (GIST):an emerging role for drug blood level testing? Cancer Treat Rev 2011;37:291-9. [Crossref] [PubMed]
- Au WY, Caguioa PB, Chuah C, et al. Chronic myeloid leukemia in Asia. Int J Hematol 2009;89:14-23. [Crossref] [PubMed]
- Ali MD, Badi AI, Al-Zebari SS, et al. Response to tyrosine kinase inhibitors in chronic myeloid leukemia: experience from a west Asian developing country. Int J Hematol 2014;100:274-80. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [Crossref] [PubMed]
- Patel S. Long-term efficacy of imatinib for treatment of metastatic GIST. Cancer Chemother Pharmacol 2013;72:277-86. [Crossref] [PubMed]
- Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase:S0033. J Clin Oncol 2008;26:626-32. [Crossref] [PubMed]
- Huang Y. Pharmacogenetics/genomics of membrane transporters in cancer chemotherapy. Cancer Metastasis Rev 2007;26:183-201. [Crossref] [PubMed]
- Takahashi T, Yamamoto N, Nukiwa T, et al. Phase II study of erlotinib in Japanese patients with advanced non-small cell lung cancer. Anticancer Res 2010;30:557-63. [PubMed]
- Thomas F, Rochaix P, White-Koning M, et al. Population pharmacokinetics of erlotinib and its pharmacokinetic/pharmacodynamic relationships in head and neck squamous cell carcinoma. Eur J Cancer 2009;45:2316-23. [Crossref] [PubMed]
- Kobayashi H, Sato K, Niioka T, et al. Relationship among gefitinib exposure, polymorphisms of its metabolizing enzymes and transporters, and side effects in Japanese patients with non-small-cell lung cancer. Clin Lung Cancer 2015;16:274-81. [Crossref] [PubMed]
- Yang L, Shi L, Fu Q, et al. Efficacy and safety of sorafenib in advanced renal cell carcinoma patients: Results from a long-term study. Oncol Lett 2012;3:935-9. [PubMed]
- Zhang H, Dong B, Lu JJ, et al. Efficacy of sorafenib on metastatic renal cell carcinoma in Asian patients: results from a multicenter study. BMC Cancer 2009;9:249. [Crossref] [PubMed]
- Kim SH, Kim S, Nam BH, et al. Efficacy and Safety of Sorafenib Therapy on Metastatic Renal Cell Carcinoma in Korean Patients: Results from a Retrospective Multicenter Study. PLoS One 2015;10:e0135165 [Crossref] [PubMed]
- Stadler WM, Figlin RA, McDermott DF, et al. Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer 2010;116:1272-80. [Crossref] [PubMed]
- Li J, Brahmer J, Messersmith W, et al. Binding of gefitinib, an inhibitor of epidermal growth factor receptor-tyrosine kinase, to plasma proteins and blood cells:in vitro and in cancer patients. Invest New Drugs 2006;24:291-7. [Crossref] [PubMed]
- Bins S, Eechoute K, Kloth JS, et al. Prospective Analysis in GIST Patients on the Role of Alpha-1 Acid Glycoprotein in Imatinib Exposure. Clin Pharmacokinet 2017;56:305-10. [Crossref] [PubMed]
- Gambacorti-Passerini C, Zucchetti M, Russo D, et al. Alpha1 acid glycoprotein binds to imatinib (STI571) and substantially alters its pharmacokinetics in chronic myeloid leukemia patients. Clin Cancer Res 2003;9:625-32. [PubMed]
- Zhong JS, Meng FY, Xu D, et al. Serum alpha1-acid glycoprotein, imatinib concentration and efficacy in chronic myeloid leukemia patients. Zhonghua Yi Xue Za Zhi 2011;91:2120-3. [PubMed]
- Feely J, Grimm T. A comparison of drug protein binding and alpha 1-acid glycoprotein concentration in Chinese and Caucasians. Br J Clin Pharmacol 1991;31:551-2. [Crossref] [PubMed]
- Jabir RS, Ho GF, Annuar MABA, et al. Plasma alpha-1-acid glycoprotein as a potential predictive biomarker for non-haematological adverse events of docetaxel in breast cancer patients. Biomarkers 2017;1-5. [Crossref] [PubMed]
- Wang J, Thornton JC, Russell M, et al. Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr 1994;60:23-8. [PubMed]
- Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 2002;3:141-6. [Crossref] [PubMed]
- Rush EC, Goedecke JH, Jennings C, et al. BMI, fat and muscle differences in urban women of five ethnicities from two countries. Int J Obes (Lond) 2007;31:1232-9. [Crossref] [PubMed]
- Lear SA, Kohli S, Bondy GP, et al. Ethnic variation in fat and lean body mass and the association with insulin resistance. J Clin Endocrinol Metab 2009;94:4696-702. [Crossref] [PubMed]
- Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet 2010;49:71-87. [Crossref] [PubMed]
- Judson I, Ma P, Peng B, et al. Imatinib pharmacokinetics in patients with gastrointestinal stromal tumour: a retrospective population pharmacokinetic study over time. EORTC Soft Tissue and Bone Sarcoma Group. Cancer Chemother Pharmacol 2005;55:379-86. [Crossref] [PubMed]
- Schmidli H, Peng B, Riviere GJ, et al. Population pharmacokinetics of imatinib mesylate in patients with chronic-phase chronic myeloid leukaemia: results of a phase III study. Br J Clin Pharmacol 2005;60:35-44. [Crossref] [PubMed]
- Widmer N, Decosterd LA, Csajka C, et al. Population pharmacokinetics of imatinib and the role of alpha-acid glycoprotein. Br J Clin Pharmacol 2006;62:97-112. [Crossref] [PubMed]
- Kawaguchi T, Hamada A, Hirayama C, et al. Relationship between an effective dose of imatinib, body surface area, and trough drug levels in patients with chronic myeloid leukemia. Int J Hematol 2009;89:642-8. [Crossref] [PubMed]
- Antoun S, Baracos VE, Birdsell L, et al. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol 2010;21:1594-8. [Crossref] [PubMed]
- Huillard O, Mir O, Peyromaure M, et al. Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. Br J Cancer 2013;108:1034-41. [Crossref] [PubMed]
- Gupta A, Jarzab B, Capdevila J, et al. Population pharmacokinetic analysis of lenvatinib in healthy subjects and patients with cancer. Br J Clin Pharmacol 2016;81:1124-33. [Crossref] [PubMed]
- Hsiao AF, Wong MD, Goldstein MS, et al. Variation in complementary and alternative medicine (CAM) use across racial/ethnic groups and the development of ethnic-specific measures of CAM use. J Altern Complement Med 2006;12:281-90. [Crossref] [PubMed]
- Arcury TA, Suerken CK, Grzywacz JG, et al. Complementary and alternative medicine use among older adults: ethnic variation. Ethn Dis 2006;16:723-31. [PubMed]
- Gan GG, Leong YC, Bee PC, et al. Complementary and alternative medicine use in patients with hematological cancers in Malaysia. Support Care Cancer 2015;23:2399-406. [Crossref] [PubMed]
- Sparber A, Bauer L, Curt G, et al. Use of complementary medicine by adult patients participating in cancer clinical trials. Oncol Nurs Forum 2000;27:623-30. [PubMed]
- Molassiotis A, Fernadez-Ortega P, Pud D, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol 2005;16:655-63. [Crossref] [PubMed]
- Kim MJ, Lee SD, Kim DR, et al. Use of complementary and alternative medicine among Korean cancer patients. Korean J Intern Med 2004;19:250-6. [Crossref] [PubMed]
- Meijerman I, Beijnen JH, Schellens JH. Herb-drug interactions in oncology: focus on mechanisms of induction. Oncologist 2006;11:742-52. [Crossref] [PubMed]
- Loquai C, Schmidtmann I, Garzarolli M, et al. Interactions from complementary and alternative medicine in patients with melanoma. Melanoma Res 2017;27:238-42. [Crossref] [PubMed]
- Shao J, Markowitz JS, Bei D, et al. Enzyme- and transporter-mediated drug interactions with small molecule tyrosine kinase inhibitors. J Pharm Sci 2014;103:3810-33. [Crossref] [PubMed]
- Stockley's Herbal Medicines Interactions [Internet]. The Pharmaceutical Press. 2009 [Accessed 21st March 2017]. Available online: http://www.medicinescomplete.com.ezproxy1.library.usyd.edu.au/
- Pithavala YK, Tortorici M, Toh M, et al. Effect of rifampin on the pharmacokinetics of Axitinib (AG-013736) in Japanese and Caucasian healthy volunteers. Cancer Chemother Pharmacol 2010;65:563-70. [Crossref] [PubMed]
- Abbas R, Boni J, Sonnichsen D. Effect of rifampin on the pharmacokinetics of bosutinib, a dual Src/Abl tyrosine kinase inhibitor, when administered concomitantly to healthy subjects. Drug Metab Pers Ther 2015;30:57-63. [Crossref] [PubMed]
- Nguyen L, Holland J, Miles D, et al. Pharmacokinetic (PK) drug interaction studies of cabozantinib: Effect of CYP3A inducer rifampin and inhibitor ketoconazole on cabozantinib plasma PK and effect of cabozantinib on CYP2C8 probe substrate rosiglitazone plasma PK. J Clin Pharmacol 2015;55:1012-23. [Crossref] [PubMed]
- Xu H, O'Gorman M, Tan W, et al. The effects of ketoconazole and rifampin on the single-dose pharmacokinetics of crizotinib in healthy subjects. Eur J Clin Pharmacol 2015;71:1441-9. [Crossref] [PubMed]
- Dasatinib. FDA.gov. U.S. Food and Drug Administration. 2010. Accessed 10 Feb 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021986s7s8lbl.pdf
- Hamilton M, Wolf JL, Drolet DW, et al. The effect of rifampicin, a prototypical CYP3A4 inducer, on erlotinib pharmacokinetics in healthy subjects. Cancer Chemother Pharmacol 2014;73:613-21. [Crossref] [PubMed]
- Swaisland HC, Ranson M, Smith RP, et al. Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol. Clin Pharmacokinet 2005;44:1067-81. [Crossref] [PubMed]
- de Jong J, Skee D, Murphy J, et al. Effect of CYP3A perpetrators on ibrutinib exposure in healthy participants. Pharmacol Res Perspect 2015;3:e00156 [Crossref] [PubMed]
- Bolton AE, Peng B, Hubert M, et al. Effect of rifampicin on the pharmacokinetics of imatinib mesylate (Gleevec, STI571) in healthy subjects. Cancer Chemother Pharmacol 2004;53:102-6. [Crossref] [PubMed]
- Smith DA, Koch KM, Arya N, et al. Effects of ketoconazole and carbamazepine on lapatinib pharmacokinetics in healthy subjects. Br J Clin Pharmacol 2009;67:421-6. [Crossref] [PubMed]
- Tanaka C, Yin OQ, Smith T, et al. Effects of rifampin and ketoconazole on the pharmacokinetics of nilotinib in healthy participants. J Clin Pharmacol 2011;51:75-83. [Crossref] [PubMed]
- Rey JB, Launay-Vacher V, Tournigand C. Regorafenib as a single-agent in the treatment of patients with gastrointestinal tumors: an overview for pharmacists. Target Oncol 2015;10:199-213. [Crossref] [PubMed]
- Shi JG, Chen X, Emm T, et al. The effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 phosphate) in healthy volunteers. J Clin Pharmacol 2012;52:809-18. [Crossref] [PubMed]
- Adams VR, Leggas M. Sunitinib malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumors. Clin Ther 2007;29:1338-53. [Crossref] [PubMed]
- Martin P, Oliver S, Robertson J, et al. Pharmacokinetic drug interactions with vandetanib during coadministration with rifampicin or itraconazole. Drugs R D 2011;11:37-51. [Crossref] [PubMed]
- Pithavala YK, Tong W, Mount J, et al. Effect of ketoconazole on the pharmacokinetics of axitinib in healthy volunteers. Invest New Drugs 2012;30:273-81. [Crossref] [PubMed]
- Abbas R, Hug BA, Leister C, et al. Effect of ketoconazole on the pharmacokinetics of oral bosutinib in healthy subjects. J Clin Pharmacol 2011;51:1721-7. [Crossref] [PubMed]
- Johnson FM, Agrawal S, Burris H, et al. Phase 1 pharmacokinetic and drug-interaction study of dasatinib in patients with advanced solid tumors. Cancer 2010;116:1582-91. [Crossref] [PubMed]
- Rakhit A, Pantze MP, Fettner S, et al. The effects of CYP3A4 inhibition on erlotinib pharmacokinetics: computer-based simulation (SimCYP) predicts in vivo metabolic inhibition. Eur J Clin Pharmacol 2008;64:31-41. [Crossref] [PubMed]
- Dutreix C, Peng B, Mehring G, et al. Pharmacokinetic interaction between ketoconazole and imatinib mesylate (Glivec) in healthy subjects. Cancer Chemother Pharmacol 2004;54:290-4. [Crossref] [PubMed]
- Tan AR, Gibbon DG, Stein MN, et al. Effects of ketoconazole and esomeprazole on the pharmacokinetics of pazopanib in patients with solid tumors. Cancer Chemother Pharmacol 2013;71:1635-43. [Crossref] [PubMed]
- Chee EL, Lim AY, Modamio P, et al. Sunitinib tissue distribution changes after coadministration with ketoconazole in mice. Eur J Drug Metab Pharmacokinet 2016;41:309-19. [Crossref] [PubMed]
- Jamal A, King BA, Neff LJ, et al. Morbidity and Mortality Weekly Report. U.S. Department of Health and Human Services. Center for Disease Control and Prevention. 2016:1206-11.
- Asma S, Mackay J, Song SY, et al. The GATS Atlas. CDC Foundation, Atlanta, 2015.
- Faber MS, Fuhr U. Time response of cytochrome P450 1A2 activity on cessation of heavy smoking. Clin Pharmacol Ther 2004;76:178-84. [Crossref] [PubMed]
- Hamilton M, Wolf JL, Rusk J, et al. Effects of smoking on the pharmacokinetics of erlotinib. Clin Cancer Res 2006;12:2166-71. [Crossref] [PubMed]
- Hamilton M, Wolf JL, Zborowski D, et al. Tarceva (erlotinib) exposure/effects (EE) analysis from a Phase III study in advanced NSCLC: Effect of smoking on the PK of erlotinib. Cancer Res 2005;65:abstr 6165.
- Zhang Y, Kang S, Fang W, et al. Impact of smoking status on EGFR-TKI efficacy for advanced non-small-cell lung cancer in EGFR mutants: a meta-analysis. Clin Lung Cancer 2015;16:144-51.e1. [Crossref] [PubMed]
- Porosnicu M, Waltonen JD, Sullivan C, et al. Pilot study to evaluate the effect of erlotinib administered before surgery in operable patients with squamous cell carcinoma of the head and neck (SCCHN). J Clin Oncol 2012;29:5568. [Crossref]
- Jiang H. Overview of gefitinib in non-small cell lung cancer: an Asian perspective. Jpn J Clin Oncol 2009;39:137-50. [Crossref] [PubMed]
- Calvo E, Baselga J. Ethnic differences in response to epidermal growth factor receptor tyrosine kinase inhibitors. J Clin Oncol 2006;24:2158-63. [Crossref] [PubMed]
- Chen YM, Perng RP, Tsai CM. Gefitinib treatment is highly effective in non-small-cell lung cancer patients failing previous chemotherapy in Taiwan: a prospective phase II study. J Chemother 2005;17:679-84. [Crossref] [PubMed]
- Lee DH, Han JY, Yu SY, et al. The role of gefitinib treatment for Korean never-smokers with advanced or metastatic adenocarcinoma of the lung: a prospective study. J Thorac Oncol 2006;1:965-71. [Crossref] [PubMed]
- Belloc F, Moreau-Gaudry F, Uhalde M, et al. Imatinib and nilotinib induce apoptosis of chronic myeloid leukemia cells through a Bim-dependant pathway modulated by cytokines. Cancer Biol Ther 2007;6:912-9. [Crossref] [PubMed]
- Tanimoto K, Yoshiga K, Eguchi H, et al. Hypoxia-inducible factor-1alpha polymorphisms associated with enhanced transactivation capacity, implying clinical significance. Carcinogenesis 2003;24:1779-83. [Crossref] [PubMed]
- Logan JE, Rampersaud EN, Sonn GA, et al. Systemic therapy for metastatic renal cell carcinoma: a review and update. Rev Urol 2012;14:65-78. [PubMed]
- Fajardo LF, Kwan HH, Kowalski J, et al. Dual role of tumor necrosis factor-alpha in angiogenesis. Am J Pathol 1992;140:539-44. [PubMed]
- Naredi PL, Lindner PG, Holmberg SB, et al. The effects of tumour necrosis factor alpha on the vascular bed and blood flow in an experimental rat hepatoma. Int J Cancer 1993;54:645-9. [Crossref] [PubMed]
- González-Galarza FF, Takeshita L, Santos E, et al. Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Res 2015;43:D784-8. [Crossref] [PubMed]
- Wang Y, Zheng Y, Zhang W, et al. Polymorphisms of KDR gene are associated with coronary heart disease. J Am Coll Cardiol 2007;50:760-7. [Crossref] [PubMed]
- Hughes TP, Hochhaus A, Branford S, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS). Blood 2010;116:3758-65. [Crossref] [PubMed]
- Branford S, Kim DW, Soverini S, et al. Initial molecular response at 3 months may predict both response and event-free survival at 24 months in imatinib-resistant or -intolerant patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase treated with nilotinib. J Clin Oncol 2012;30:4323-9. [Crossref] [PubMed]
- Hanfstein B, Muller MC, Hehlmann R, et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML). Leukemia 2012;26:2096-102. [Crossref] [PubMed]
- Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia:3-year follow-up from a randomized phase 3 trial (DASISION). Blood 2014;123:494-500. [Crossref] [PubMed]
- Papadantonakis N, Cassaday RD, Liedtke M, et al. Prognostic parameters in adults with acute lymphoblastic leukemia at second complete response. American Society of Haematology 58th Annual Meeting 2016:1603.
- Fujisawa S, Mizuta S, Akiyama H, et al. Phase II study of imatinib-based chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia. Am J Hematol 2017;92:367-74. [Crossref] [PubMed]
- Bilgi N, Bell K, Ananthakrishnan AN, et al. Imatinib and Panax ginseng: a potential interaction resulting in liver toxicity. Ann Pharmacother 2010;44:926-8. [Crossref] [PubMed]
- Frye RF, Fitzgerald SM, Lagattuta TF, et al. Effect of St John's wort on imatinib mesylate pharmacokinetics. Clin Pharmacol Ther 2004;76:323-9. [Crossref] [PubMed]
- Moore LB, Goodwin B, Jones SA, et al. St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci U S A 2000;97:7500-2. [Crossref] [PubMed]
- Smith PF, Bullock JM, Booker BM, et al. Induction of imatinib metabolism by hypericum perforatum. Blood 2004;104:1229-30. [Crossref] [PubMed]
- Hwang SW, Han HS, Lim KY, et al. Drug interaction between complementary herbal medicines and gefitinib. J Thorac Oncol 2008;3:942-3. [Crossref] [PubMed]
- Ge J, Tan BX, Chen Y, et al. Interaction of green tea polyphenol epigallocatechin-3-gallate with sunitinib: potential risk of diminished sunitinib bioavailability. J Mol Med (Berl) 2011;89:595-602. [Crossref] [PubMed]
- Yang CS, Pan E. The effects of green tea polyphenols on drug metabolism. Expert Opin Drug Metab Toxicol 2012;8:677-89. [Crossref] [PubMed]
- Maher HM, Alzoman NZ, Shehata SM, et al. UPLC-ESI-MS/MS study of the effect of green tea extract on the oral bioavailability of erlotinib and lapatinib in rats: Potential risk of pharmacokinetic interaction. J Chromatogr B Analyt Technol Biomed Life Sci 2017;1049-1050:30-40. [Crossref] [PubMed]
- Knop J, Misaka S, Singer K, et al. Inhibitory Effects of Green Tea and (-)-Epigallocatechin Gallate on Transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2-K and P-Glycoprotein. PLoS One 2015;10:e0139370 [Crossref] [PubMed]



