
The value of pre- and post-contrast-enhanced ultrasound in evaluation of malignant potential of gastrointestinal stromal tumors
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common type of mesenchymal tumor of the gastrointestinal tract (1). The symptoms of GISTs are variable, and they are often detected incidentally during imaging examinations. All GISTs have some degree of malignant potential. Accurate risk stratification of GISTs became a focus of clinical interest because of advances in systemic adjuvant treatments. Recently, imaging modalities have been investigated to assess the malignant potential of GISTs preoperatively (2-5). Some research indicates that angiogenesis and expression of vascular endothelial growth factor (VEGF) play important roles in the prognosis of GISTs (6,7). Therefore, evaluation of intratumoral vascularity has been another approach to assess the malignancy risk of GISTs.
Trans-abdominal ultrasound (TAUS) is a well-tolerated, non-invasive, easily accessible and radiation-free imaging modality. It enables real-time visualization of bowel movement, and is widely applied in initial diagnosis and follow-up monitoring of a variety of gastrointestinal diseases (8,9). TAUS can help to detect GISTs with exophytic growth patterns or those located in the lower digestive tract, which are difficult to detect using endoscopy. Contrast-enhanced ultrasound (CEUS) allows evaluation of microvascularization with high spatial resolution. Furthermore, perfusion parameters obtained from time-intensity curves can provide information on blood volume and velocity, which is helpful for accurately assessing tumor angiogenesis (10-12). The aim of this study was to analyze perfusion patterns in patients with GISTs using CEUS, to compare conventional ultrasound (US) and CEUS findings with pathology results, and to investigate whether conventional US and CEUS can predict the malignancy risk of GISTs.
Methods
Patients
From February 2014 to January 2016, a total of 50 consecutive patients with pathology-confirmed primary GISTs from the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College, were enrolled in this study. Before surgery all the patients underwent conventional US examinations, and 44 of them underwent CEUS examinations. Patients with gastric and small intestinal tumors underwent TAUS; patients with rectal tumors underwent endorectal ultrasound (ERUS). The study was approved by the ethics committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College. The ID of the approval is NCC2013S-006. Each patient gave informed consent.
TAUS examinations
All TAUS examinations were performed using a Philips iU22 unit (Philips Medical Systems, Bothell, WA, USA), with a broadband curved array transducer (C5-2). After an overnight fast, the patient was placed in a supine position and given 500–700 mL water to drink, in order to fill the stomach or small intestine. The US examination was initiated about 30 seconds after water ingestion. The tumor was evaluated for tumor size, margin, shape, location in the GI tract, echogenicity, and the existence of cystic areas, calcification, and intratumoral gas.
ERUS examinations
All ERUS examinations were performed using a Philips iU22 unit (Philips Medical Systems), with an end-fire type of endorectal probe (C5-9 sec). The patient was placed in a left lateral decubitus position and prepared with an enema prior to the examination. About 100–150 mL of coupling gel was injected into the rectum to fill the rectal lumen, to ensure that the five layers of the bowel wall and the tumor could be seen clearly (13). The tumor was evaluated for size, margin, shape, echogenicity, and the existence of cystic areas, calcification, and intratumoral gas.
CEUS examinations
CEUS examination was performed immediately after the conventional US examination. The mechanical index was 0.08–0.11, with the focus placed under the region of interest (ROI). A total of 2.4 mL of the contrast agent SonoVue (Bracco Imaging, Milan, Italy) was administered into a peripheral vein in bolus, through a 20-gauge intravenous cannula, followed by a flush of 5 mL of 0.9% saline solution. The examinations were recorded for 3 minutes, and the data was then stored in a DICOM file. The arterial phase was defined as the first 30 seconds following contrast agent administration, and the venous phase as the period 30–60 seconds after administration. In the arterial phase, the presence/absence of large and irregular vessels was assessed, as well as the presence/absence of non-enhancing areas. Patterns of enhancement were categorized as homogeneous or heterogeneous. The DICOM data from the CEUS was analyzed using QLab software (version 9.1; Philips Medical Systems, Bothell, WA, USA), and regions of interest (ROI) were drawn at most enhanced area. A time-intensity curve was constructed for each ROI, and perfusion parameters including arrival time (AT), time to peak enhancement (TTP), enhanced intensity (EI), and area under the curve (AUC) were derived from it. The AT was defined as the time from injection to the beginning of enhancement. The TTP was defined as the time from injection to peak enhancement. The EI was defined as peak intensity minus baseline intensity. All the imaging data were analyzed by two experienced radiologists who were blinded to the final diagnosis.
Histopathological analysis
Histological sections were examined by an experienced pathologist. The malignant potential of GISTs was classified as high-, intermediate- and low-risk based on tumor size, tumor site, mitotic count, and presence of tumor rupture, in accordance with the widely accepted NIH 2008 risk stratification criteria for GISTs (14).
Statistical analysis
Quantitative data are presented as means ± standard deviations (SD), whereas categorical data are presented as rates and proportions. Statistical analyses of the differences between pre- and post-CEUS among the three groups were conducted using a one-way analysis of variance or a Chi-square test. Spearman correlation analysis was used to investigate the correlation between EI and GIST risk. Two-sided P values <0.05 are considered statistically significant. All statistical analyses were performed using SPSS version 19.9 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
Fifty patients were diagnosed with GISTs, including 5 with low risk, 9 with intermediate risk, and 36 with high risk. The mean age was 55.1±10.5 years, ranging from 27–71 years. The male:female ratio was 1.5:1. The GIST tumors had an average size of 11.4±5.5 cm, ranging from 1.8–31 cm. Twenty-five of the GISTs were located in the stomach, 16 in the small intestine, 5 in the rectum, and 4 in the peritoneum or mesentery.
Conventional US findings
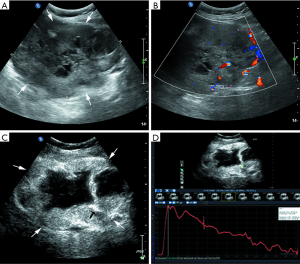
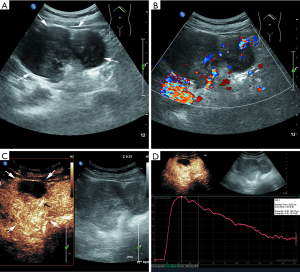
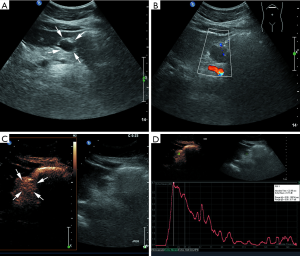
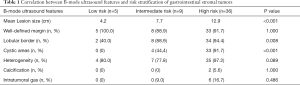
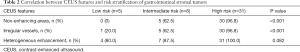
Most lesions were larger than 5 cm (44 patients, 88%) and appeared as well-defined heterogeneous, hypoechoic extraluminal masses. The high risk GIST typically appeared as a lobular, cystic-solid mass with rich blood flow (Figure 1A,B), and CEUS showed rapid intense arterial enhancement followed by gradual washout (Figure 1C,D). The intermediate risk GIST also often appeared as a lobular, cystic-solid or solid mass with rich blood flow (Figure 2A,B), and CEUS showed rapid intense arterial enhancement followed by gradual washout (Figure 2C,D). The low risk GIST usually appeared as a round or ovoid solid mass with no vascularization (Figure 3A,B), and CEUS showed rapid moderate arterial enhancement followed by gradual washout (Figure 3C,D). Thirty-seven patients (74%) had lesions with large centrally located cystic areas or small patchy cystic areas. Intratumoral gas was detected in nine patients (18%), appearing as hyperechoic foci with posterior acoustic shadowing, which was caused by communication with the bowel loops, sometimes mimicking a pseudokidney or presenting a target-like appearance. Table 1 shows the US features of the GISTs by risk category. Higher risk was associated with larger lesion size, lobular border, and cystic areas (P<0.05), but not with margin, heterogeneity, calcification or intratumoral gas (P>0.05).
Full table
CEUS findings
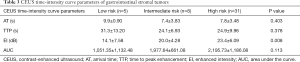
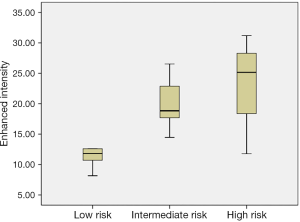
Forty-four patients with GISTs underwent CEUS examinations immediately after the conventional US examinations. A detailed description of the CEUS results appears in Tables 2 and 3. The presence of irregular vessels and non-enhancing areas in CEUS was significantly correlated with risk classification (P<0.001) (Table 2). Non-enhancing areas were most common in the high-risk group (96.8%, 30 of 31), followed by the intermediate-risk group (62.5%, 5 of 8) and the low-risk group (0%, 0 of 5). Intratumoral irregular vessels appeared most frequently in the high-risk group (96.8%, 30 of 31), followed by the intermediate-risk group (62.5%, 5 of 8) and the low-risk group (20%, 1 of 5). Every GIST showed a rapid heterogeneous or homogeneous enhancement in the arterial phase, followed by a gradual washout in the venous phase. We also assessed the correlation of CEUS perfusion parameters with GIST risk category (Table 3). The EI measure was higher in the high and intermediate risk groups than in the low risk group (P=0.008), and the EI showed a significant positive correlation with the GIST risk category (r=0.393, P=0.008) (Figure 4). There were no statistically significant differences in AT, TTP or AUC among the three groups.
Full table
Full table
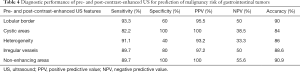
Based on the results shown in Tables 2 and 3, we calculated the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of all the conventional US and CEUS features (Table 4), by dividing the patients into high grade GIST (high-and intermediate-risk) and low grade GIST groups.
Full table
Discussion
GISTs are the most common mesenchymal tumors in the gastrointestinal tract. Sixty percent of GISTs occur in the stomach, 30% in the small intestine, and less than 5% in the large bowel. In contrast to myogenic or neurogenic tumors, GISTs are driven by oncogenic, mutational gain-of-function activation of KIT or platelet-derived growth factor receptor alpha (PDGFRα). Most GISTs express KIT (CD117), DOG1 and CD34 proteins. GISTs have variable biological behavior, with a wide spectrum of malignant potential, depending on tumor size, site, and mitosis count (1). That predicting the prognosis of GISTs remains challenging. Pathological specimens obtained by endoscopic ultrasound (EUS)-guided fine needle aspiration are usually insufficient for evaluating the mitotic count and sometimes are associated with complications (15). Some studies have reported that high intratumoral microvessel density and high levels of expression of VEGF were correlated with poor prognosis (16). Imaging of microvascularization may be a complementary technique for predicting the prognosis of GISTs. In contrast to computed tomography (CT) and magnetic resonance imaging (MRI) contrast agents; the US contrast agent is a strict blood pool agent, which is optimal for evaluation of tumor vascularity. Moreover, perfusion-related parameters allow quantitative analysis of the degree and pattern of enhancement.
Transabdominal US is considered to be a first-line tool for assessing patients with gastrointestinal symptoms. Futagami et al. reported that when transabdominal US was used, the detection rate of gastric submucosal tumors over 20 mm in size was 97–100% (17). In the present study, using transabdominal US, we detected tumors with size ranging from 2.8 to 31 cm. In our previous study, we also detected 53 GISTs by transabdominal US, with size ranging from 2.0–19.9 cm (18). Sugihara et al. reported three cases of medium-sized exophytic GISTs that were detected initially by transabdominal US while they were invisible in endoscopy (19). Thus transabdominal US may be useful for detecting endoscopically invisible medium or large sized exophytic GISTs.
Some studies have reported that certain EUS features are correlated with the malignant potential of GISTs, including a large tumor size (>3–5 cm), irregular extraluminal border, intratumoral echogenic foci, and cystic areas (20,21). Our study found that transabdominal US could be helpful in assessment of the malignancy risk of GISTs. A lobular border and cystic areas were more common among the high and intermediate risk groups, which is consistent with EUS findings. Although the presence of intratumoral gas did not have significant difference among the three risk groups, this feature was observed only in the high risk group. The intratumoral gas was caused by fistulization into the bowel lumen, which occurred more frequently in large tumors with massive necrosis.
Several studies have attempted to evaluate the association between contrast-enhanced harmonic endoscopic ultrasound (CH-EUS) findings and the degree of malignancy or mitotic rate in small samples of patients with GISTs. Yamashita et al. found an association between the presence of intratumoral vessels and an increased risk of malignancy (22). The intratumoral vessels observed in CH-EUS corresponded to large vessels with a diameter >500 µm by histological analysis, and most of them lacked elastic fibers, indicating that they resulted from neovascularization. Sakamoto et al. reported that irregular vessels and heterogeneous enhancement in CEH-EUS were seen more frequently in high risk GIST patients (3). Fukuta et al. found that all five intermediate and high risk GISTs showed rich vascularity, while five of eight low-risk GISTs showed poor vascularity in transabdominal CEUS (2). Conflicting results have, however, been reported. Park et al. found that there were no significant differences in the presence of irregular vessels or in parenchymal perfusion between low and high grade GISTs (23).
To the best of our knowledge, the association between risk stratification and various CEUS features has not been thoroughly investigated in any large study. Our findings show that avascular areas and irregular vessels appeared in CEUS for a high percentage of intermediate- and high-risk GISTs. There was no significant difference in heterogeneous enhancement among different risk categories. Another finding was that the EI was associated with risk stratification. Image intensity is in direct correlation with capillary bed volume; thus, increased EI corresponds with increased microvascularization and poor prognosis. This result suggests that the EI provide additional information in evaluation of the malignant potential of GISTs. On the other hand, AT, time to peak and AUC were not correlated with tumor risk. We also evaluated sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for pre- and post- CEUS features for differentiating high grade GISTs from low grade GISTs. In our study, the presence of a lobular border and heterogeneous echotexture showed high sensitivity but low specificity, whereas the presence of non-enhancing areas in CEUS had good performance characteristics (sensitivity of 89.7% and specificity of 100%). However, all the US findings have low negative predictive value, suggesting that neither pre- nor post-CEUS could identify low risk GISTs very accurately.
The main limitations in this study are that it was a single center study with a relatively small number of patients and didn’t include enough GIST patients with low and intermediate malignant potential. In conclusion, this study investigated pre- and post-CEUS examinations of GISTs, and compared various features among different risk groups. The presence of a lobular border, cystic areas on US, and irregular vessels and non-enhancing areas on CEUS, correlated with higher malignant potential of the GISTs. The pre- and post-CEUS can provide additional information for risk stratification in gastrointestinal tumors, but need to be investigated in larger patient populations.
Acknowledgments
Funding: This work was supported by Beijing Municipal Science & Technology Commission (No. Z131107002213016); Beijing Hope Run Special Fund of China Cancer Research Foundation (CCRF) (No. LC2016A04); Peking Union Medical College (PUMC) Youth Fund and the Fundamental Research Funds for the Central Universities (No. 2017320015); Wu Jieping medical foundation (320675012622); Chinese Academy of Medical Sciences Innovation Fund for Medical Science (2017-I2M-1-006).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Translational Imaging in Cancer Patient Care”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.10.18). The series “Translational Imaging in Cancer Patient Care” was commissioned by the editorial office without any funding or sponsorship. Yong Wang served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College. The ID of the approval is NCC2013S-006. Each patient gave informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reichardt P, Hogendoorn PC, Tamborini E, et al. Gastrointestinal stromal tumors I: pathology, pathobiology, primary therapy, and surgical issues. Semin Oncol 2009;36:290-301. [Crossref] [PubMed]
- Fukuta N, Kitano M, Maekawa K, et al. Estimation of the malignant potential of gastrointestinal stromal tumors: the value of contrast-enhanced coded phase-inversion harmonics US. J Gastroenterol 2005;40:247-55. [Crossref] [PubMed]
- Sakamoto H, Kitano M, Matsui S, et al. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos). Gastrointest Endosc 2011;73:227-37. [Crossref] [PubMed]
- Hasegawa S, Semelka RC, Noone TC, et al. Gastric stromal sarcomas: correlation of MR imaging and histopathologic findings in nine patients. Radiology 1998;208:591-5. [Crossref] [PubMed]
- Hersh MR, Choi J, Garrett C, et al. Imaging gastrointestinal stromal tumors. Cancer Control 2005;12:111-5. [Crossref] [PubMed]
- Takahashi R, Tanaka S, Kitadai Y, et al. Expression of vascular endothelial growth factor and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncology 2003;64:266-74. [Crossref] [PubMed]
- Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol 2006;39:469-78. [PubMed]
- Ricci R, Bontempo I, Corazziari E, et al. Real time ultrasonography of the gastric antrum. Gut 1993;34:173-6. [Crossref] [PubMed]
- Nylund K, Ødegaard S, Hausken T, et al. Sonography of the small intestine. World J Gastroenterol 2009;15:1319-30. [Crossref] [PubMed]
- Wang Y, Li L, Wang YX, et al. Time-intensity curve parameters in rectal cancer measured using endorectal ultrasonography with sterile coupling gels filling the rectum: correlations with tumor angiogenesis and clinicopathological features. Biomed Res Int 2014;2014:587806 [PubMed]
- Pysz MA, Guracar I, Tian L, et al. Fast microbubble dwell-time based ultrasonic molecular imaging approach for quantification and monitoring of angiogenesis in cancer. Quant Imaging Med Surg 2012;2:68-80. [PubMed]
- Wáng YX, Choi Y, Chen Z, et al. Molecular imaging: from bench to clinic. Biomed Res Int 2014;2014:357258 [PubMed]
- Wang Y, Zhou CW, Hao YZ, et al. Improvement in T-Staging of Rectal Carcinoma: Using a Novel Endorectal Ultrasonography Technique with Sterile Coupling Gel Filling the Rectum. Ultrasound Med Biol 2012;38:574-9. [Crossref] [PubMed]
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. [Crossref] [PubMed]
- Polkowski M, Gerke W, Jarosz D, et al. Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: A prospective study. Endoscopy 2009;41:329-34. [Crossref] [PubMed]
- Basilio-de-Oliveira RP, Pannain VL. Prognostic angiogenic markers (endoglin, VEGF, CD31) and tumor cell proliferation (Ki67) for gastrointestinal stromal tumors. World J Gastroenterol 2015;21:6924-30. [Crossref] [PubMed]
- Futagami K, Hata J, Haruma K, et al. Extracorporeal ultrasound is an effective diagnostic alternative to endoscopic ultrasound for gastric submucosal tumours. Scand J Gastroenterol 2001;36:1222-6. [Crossref] [PubMed]
- Cui NY, Liu JY, Wang Y, et al. Contrast enhanced ultrasound guided biopsy shows higher positive sampling rate than conventional ultrasound guided biopsy for gastrointestinal stromal tumors diagnosis. Transl Cancer Res 2016;5:152-9. [Crossref]
- Sugihara T, Koda M, Tanimura T, et al. A report of three cases of exophytic gastrointestinal stromal tumor detected by transabdominal ultrasound. J Med Ultrason (2001) 2016;43:107-11. [Crossref] [PubMed]
- Palazzo L, Landi B, Cellier C, et al. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut 2000;46:88-92. [Crossref] [PubMed]
- Shah P, Gao F, Edmundowicz SA, et al. Predicting malignant potential of gastrointestinal stromal tumors using endoscopic ultrasound. Dig Dis Sci 2009;54:1265-9. [Crossref] [PubMed]
- Yamashita Y, Kato J, Ueda K, et al. Contrast-enhanced endoscopic ultrasonography can predict a higher malignant potential of gastrointestinal stromal tumors by visualizing large newly formed vessels. J Clin Ultrasound 2015;43:89-97. [Crossref] [PubMed]
- Park HY, Jeon SW, Lee HS, et al. Can contrast-enhanced harmonic endosonography predict malignancy risk in gastrointestinal subepithelial tumors? Endosc Ultrasound 2016;5:384-9. [Crossref] [PubMed]


