
Preliminary study on the role of serum PECAM-1 in metastatic breast cancer
Introduction
Breast cancer takes the highest prevalence and the second cause of mortality in all types of female malignant tumors (1). Despite advanced treatments, metastatic breast cancer (MBC) patients continue to exhibit poor survival. The alleviated rate of the first-line therapy for MBC patients is distinct, which depends on several factors. Thus, identifying highly sensitive and specific biomarkers for predicting the outcome of the palliative therapy would be beneficial in clinical decisions for MBC patients.
The development of early relapse or metastasis of malignant tumors partially relies on the abnormal activity of vessels in the body. Platelet and endothelial cell adhesion molecule-1 (PECAM-1), also commonly known as CD31, is a protein encoded by this gene and has been found on the surface of platelets, monocytes, neutrophils, and some types of T-cells, constituting a large portion of endothelial cells’ intercellular junctions. It is a member of the immunoglobulin superfamily and is likely involved in leukocyte migration, angiogenesis, and integrin activation (2). PECAM-1 is one of the vital factors participating in cell adhesion and angiogenesis during tumor metastasis. Several studies demonstrated that the high expression of PECAM-1 in tumors could be related to the poor outcome in malignant tumors, such as non-small-cell lung cancer, colorectal cancer (3,4), and breast cancer (5,6). The heterogeneity of tumor angiogenesis and progression have been demonstrated in transgenic mice during mammary cancer progression (7). However, little has been reported on the evaluation of the serum level of PECAM-1 in MBC patients.
Our preliminary study found that the serum level of PECAM-1 and IGF-1 would decrease after the treatment with bisphosphonates (BPs) in MBC patients; the reduction of IGF-1 was more significant in breast cancer patients who received hormonotherapy previously (8). Based on the properties of PECAM-1 and our findings of the changes in the serum level of PECAM-1 in MBC patients, we speculated an association of the serum level of PECAM-1 with the prognosis of MBC patients. We examined the serum level of PECAM-1 in those patients just diagnosed as MBC, before any-palliative therapy. The correlation between the serum level of PECAM-1 and pathological and physiological factors of MBC patients have also been studied.
Methods
Study population and design
Thirty female patients with initial diagnosis of relapse or MBC at the Zhejiang Cancer Hospital were enrolled in our study. The blood samples from patients were withdrawn from April 2010 to December 2014 and followed-up until October 2016. The median follow-up was 78 months. MBC was diagnosed according to the pathological biopsy, and the peripheral blood samples and history were assimilated simultaneously. Patients complicated with the presence of diabetes accepted the hypoglycemic therapy regularly. The peripheral blood tests included a complete blood count and serum blood biochemistry, i.e., fasting glucose, serum triglycerides (TG), total cholesterol (TC), low-density lipoprotein (LDL), and tumor markers (CEA, CA125, and CA153). The first-line palliative therapy included chemotherapy, endocrine therapy, anti-Her-2-overexpression therapy, local radiation for chest lesion, and BPs for bone metastasis. The follow-up was conducted by regular-outpatient-review and telephone calls to each patient since the diagnosis of relapse or metastasis post-surgery. Progression-free survival (PFS) was defined as the time of diagnosis of MBC until progress from first-line palliative therapy. Overall survival (OS) was defined as the time of MBC diagnosis until death from any cause, or the end of follow-up, whichever occurred first. The study was approved by an independent Ethics Committee.
Serum PECAM-1, glucose, and lipid estimations
Peripheral venous blood samples (5 mL) were collected before any treatment and maintained at room temperature (approximately 25 °C) for 30 min to allow clotting. Then, the samples were immediately (within 5 min) centrifuged at 3,000 ×g for 5min. The obtained serum supernatants were aliquoted and stored at −80 °C until further assessment. Serum levels of PECAM-1 were assayed using a solid-phase sandwich enzyme-linked immunosorbent assay (ELISA, Quantikine Immunoassays R&D Systems, France) in duplicate, according to the manufacturer’s instructions.
Statistical analysis
The data were analyzed using the SPSS software version 15.0 (SPSS Inc., Chicago, IL, USA). The Kaplan-Meier method and Cox regression analysis were used for determining PFS and OS rates. Other data were analyzed by the Chi-square test and t-test.
Results
Patients’ information
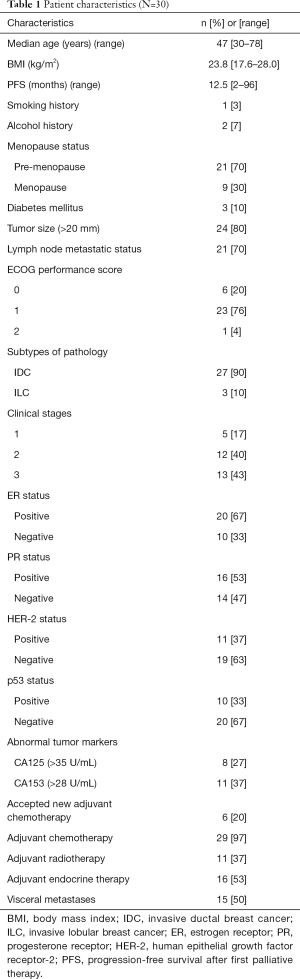
Table 1 summarizes the characteristics of patients enrolled in the present study. Thirty female MBC patients were included: 27 patients (90%) exhibited invasive ductal breast carcinoma, and 9 patients (30%) were menopausal. Three patients (10%) presented diabetes mellitus and administered medication regularly. Half of the patients had visceral metastases. All patients accepted adjuvant therapy after surgery, according to the NCCN guidelines for breast cancer. The study was approved by the medical ethics committee of Zhejiang Cancer Hospital, Hangzhou. Written informed consent was obtained from all patients prior to enrollment. The trial is registered through the Hospital Pharmaceutical Research Fund Project of Zhejiang Provincial Pharmaceutical Association, number 2016ZYY12.
Full table
Comparison between subgroups of serum PECAM-1 level in MBC patients
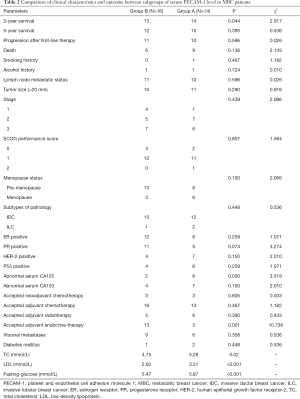
According to the median serum level of PECAM-1, patients were divided into two subgroups, Group A (<4,058.89 pg/mL) and Group B (>4,058.89 pg/mL).
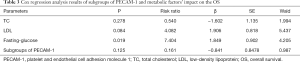
The 3-year survival of MBC patients was higher in Group A than Group B (P=0.044). A significant difference was not seen in Groups A and B either with respect to PFS or OS (P>0.05). More patients in Group B accepted the adjuvant endocrine therapy as compared to Group A patients (P=0.001). The serum TC, LDL, and fasting glucose were higher in Group A than Group B patients (P=0.02, P<0.001, and P<0.001, respectively). The serum CA125 level was higher in Group A than Group B patients (P=0.03) (Table 2). After adjusting the values of TC, LDL, and fasting glucose in the groups, Cox regression analysis showed that fasting glucose was an independent factor influencing the prognosis of MBC patients (P=0.019) (Table 3).
Full table
Full table
Discussion
Here, we explored the preliminary role of serum PECAM-1 levels in the initial recurrence or metastasis of breast cancer patients. A high level of serum PECAM-1 was found to be correlated with a poor 3-year survival in patients with MBC. Since the treatment of MBC is rather challenging, the prognosis is rather unsatisfactory. In addition, factors such as molecular subtypes and characteristics of the primary tumor, the postoperative systematic adjuvant therapies, and response to palliative therapy could impact the outcome of MBC. Therefore, the prediction and evaluation of the prognosis of MBC poses a pressing concern for clinicians.
Previous studies showed that PECAM-1 is a pivotal marker for assessing the density of microvessels and angiogenesis in vivo and in vitro (9,10). The blood vessel invasion was marked using PECAM-1 in tumor cells, thereby indicating a robust correlation of the aggressive subtypes and poor prognosis in breast cancer patients (11). A high PECAM-1 immunoreactivity was substantially related to the formation of tubules, histological grade of malignancy, and clinical stage (12). The tumor-associated macrophages (TAMs) accumulate in various cancers including breast cancer and promote tumor angiogenesis and metastasis with high specificity. PECAM-1 was reported as one of the significantly overexpressed proangiogenic factors in TAMs (13). The abnormal change in the density of microvessels was correlated to the tumor microenvironment, thereby inducing relapse or metastasis of the malignant tumor. In our study, since a limited number of biopsies were available from metastatic lesions of patients with MBC, tissue specimens from only 11 patients could be utilized in IHC for analyzing the expression of CD31. Although the paraffin sections were found to be sensitive and superior in cancer research, the CD31 staining was affected by the inflammatory background of cells and frequent loss of antigen due to fixatives containing acetic acid. Furthermore, the anti-CD31 antibodies might incorrectly render a prominent inflammatory infiltration region, such as a rich vascular spot, at low magnification (14). Considering the defects associated with monitoring the tumor tissue markers and the heterogeneity of the tissue, identifying novel peripheral blood tumor markers for early diagnosis is imperative for the adequate palliative treatment of MBC patients.
Furthermore, we found that high serum PECAM-1 level in patients proposed their candidature for the adjuvant endocrine therapy. However, only a few studies emphasized the potential crosstalk between PECAM-1 and hormone therapy. Moreover, the putative effect of PECAM-1 in hormone receptor-positive breast cancer patients calls necessitates further exploration. According to the current results, PECAM-1 is not only related to the prognosis of MBC but also associated with the metabolisms of lipid and glucose. Blood lipid and glucose are closely linked to the development of breast cancer (15,16). Increasing evidence shows that the abnormal levels of serum lipids and glucose impact the survival of breast cancer patients (17-20). Metabolic syndrome could increase the risk of breast cancer and influence the prognosis in patients (21). Also, the metabolic disturbances would impact the response and resistance to aromatase inhibitors (22). A mechanism underlying these associations might encompass chronic inflammation and insulin resistance that in turn, might drive atherogenesis, cellular proliferation, and angiogenesis (16). However, recent negative results on the fasting blood lipids or glucose might affect the survival of breast cancer patients (23,24). In the current study, fasting glucose was found to be an independent factor on the OS of the MBC patients. This conclusion was in agreement with the previous studies in MBC patients with diabetes; however, supplementary evidence should be assimilated before deeming that high fasting glucose was a negative prognostic factor for OS in MBC patients.
In the present study, a negative correlation was established between the serum level of PECAM-1 and TC, LDL, fasting glucose, and CA125. Serum PECAM-1 was considered to be involved in the inflammatory events, which induced diabetic complications, such as diabetic retinopathy (25). Furthermore, we speculated that PECAM-1 could potentially be an early marker that can indicate the blood lipid and glucose metabolism disorder and the difference in the levels of these biochemicals from that of the baseline. A previous study on the influence of microvessel density on ovarian carcinogenesis showed that the change in CA125 did not associate with the CD31 counts, thereby suggesting a sophisticated correlation between tumor vascularity, metastasis, and response to treatment (26). The recent ASCO guidelines recommended that CEA, CA15-3, and CA27.29, except CA125, could be employed as MBC biomarkers (27). Nevertheless, the value of CA125 in MBC diagnosis and prediction is yet obscure and controversial.
Although the present preliminary study consisted of a small sample size, it focused on the predictive value of serum PECAM-1 in the correction of the physiological and pathological factors in MBC patients. However, single-center and retrospective nature of the study in MBC patients are a few limitations. Therefore, in future studies, we would consider comparing the expression of PECAM-1 in the metastatic lesions with serum level of the protein. Additional combinations of peripheral biomarkers would be used to predict the prognosis of MBC patients, even integrating with PECAM-1 to establish a prognostic panel.
Conclusions
High serum level of PECAM-1 is correlated to the decline of 3-year survival of MBC patients. Further research is warranted for the evaluation of serum PECAM-1 as a prognostic biomarker in MBC patients.
Acknowledgments
Funding: This work was supported by the Zhejiang Province Medical Science Fund Project of China (2015KYB052, 2013ZA020, 2013KYB034), Zhejiang medicine institute special scientific research projects of hospital pharmacy (2016ZYY12), Zhejiang Province Medical Science Research Foundation of China (2015PYA001), Clinical research fund of Zhejiang Medical Association (2016ZYC-A06) and the Science and Technology Department of Zhejiang Province (2013C33205).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.11.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Zhejiang Cancer Hospital Ethic Institution Office (ID was 2016ZYY12). Written informed consent was obtained from all patients prior to enrollment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stewart BW, Wild CP, editors. World Cancer Report 2014. Lyon, France: International Agency for Research on Cancer, 2014.
- Mei H, Campbell JM, Paddock CM, et al. Regulation of endothelial cell barrier function by antibody-driven affinity modulation of platelet endothelial cell adhesion molecule-1 (PECAM-1). J Biol Chem 2014;289:20836-44. [Crossref] [PubMed]
- Giatromanolaki A, Koukourakis M, Sivridis E, et al. Vascular density analysis in colorectal cancer patients treated with vatalanib (PTK787/ZK222584) in the randomised CONFIRM trials. Br J Cancer 2012;107:1044-50. [Crossref] [PubMed]
- Kuang BH, Wen XZ, Ding Y, et al. The prognostic value of platelet endothelial cell adhesion molecule-1 in non-small-cell lung cancer patients. Med Oncol 2013;30:536. [Crossref] [PubMed]
- García-Quiroz J, Rivas-Suárez M, García-Becerra R, et al. Calcitriol reduces thrombospondin-1 and increases vascular endothelial growth factor in breast cancer cells: Implications for tumor angiogenesis. J Steroid Biochem Mol Biol 2014;144:215-22. [Crossref] [PubMed]
- Gu JW, Young E, Patterson SG, et al. Postmenopausal obesity promotes tumor angiogenesis and breast cancer progression in mice. Cancer Biol Ther 2011;11:910-7. [Crossref] [PubMed]
- Smith MJ, Berger RW, Minhas K, et al. Heterogeneity of vascular and progenitor cell compartments in tumours from MMTV-PyVmT transgenic mice during mammary cancer progression. Int J Exp Pathol 2011;92:106-16. [Crossref] [PubMed]
- Wang Z, Lei L, Cai XJ, et al. A preliminary study of pamidronic acid downregulation of angiogenic factors IGF-1/PECAM-1 expression in circulating level in bone metastatic breast cancer patients. Onco Targets Ther 2016;9:3147-52. [Crossref] [PubMed]
- Bimonte S, Barbieri A, Palma G, et al. Dissecting the role of curcumin in tumour growth and angiogenesis in mouse model of human breast cancer. Biomed Res Int 2015;2015:878134 [PubMed]
- Li L, Wang K, Sun X, et al. Parameters of dynamic contrast-enhanced MRI as imaging markers for angiogenesis and proliferation in human breast cancer. Med Sci Monit 2015;21:376-82. [Crossref] [PubMed]
- Klingen TA, Chen Y, Stefansson IM, et al. Tumour cell invasion into blood vessels is significantly related to breast cancer subtypes and decreased survival. J Clin Pathol 2017;70:313-9. [Crossref] [PubMed]
- Carvalho MI, Guimarães MJ, Pires I, et al. EGFR and microvessel density in canine malignant mammary tumours. Res Vet Sci 2013;95:1094-9. [Crossref] [PubMed]
- Kim OH, Kang GH, Noh H, et al. Proangiogenic TIE2+/CD31+ macrophages are the predominant population of tumor-associated macrophages infiltrating metastatic lymph nodes. Mol Cells 2013;36:432-8. [Crossref] [PubMed]
- Nico B, Maruotti N, Mangieri D, et al. Evaluation of microvascular density in tumors, pro and contra. Histol Histopathol 2008;23:601-7. [PubMed]
- Simpson ER, Brown KA. Minireview: obesity and breast cancer: a tale of inflammation and dysregulated metabolism. Mol Endocrinol 2013;27:715-25. [Crossref] [PubMed]
- Belardi V, Gallagher EJ, Novosyadlyy R, et al. Insulin and IGFs in obesity-related breast cancer. J Mammary Gland Biol Neoplasia 2013;18:277-89. [Crossref] [PubMed]
- Contiero P, Berrino F, Tagliabue G, et al. Fasting blood glucose and long-term prognosis of non-metastatic breast cancer: a cohort study. Breast Cancer Res Treat 2013;138:951-9. [Crossref] [PubMed]
- Minicozzi P, Berrino F, Sebastiani F, et al. High fasting blood glucose and obesity significantly and independently increase risk of breast cancer death in hormone receptor-positive disease. Eur J Cancer 2013;49:3881-8. [Crossref] [PubMed]
- Villarreal-Garza C, Shaw-Dulin R, Lara-Medina F, et al. Impact of diabetes and hyperglycemia on survival in advanced breast cancer patients. Exp Diabetes Res 2012;2012:732027 [PubMed]
- Berrino F, Villarini A, Traina A, et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat 2014;147:159-65. [Crossref] [PubMed]
- Chen Y, Wen YY, Li ZR, et al. The molecular mechanisms between metabolic syndrome and breast cancer. Biochem Biophys Res Commun 2016;471:391-5. [Crossref] [PubMed]
- Schech A, Yu S, Goloubeva O, et al. A nude mouse model of obesity to study the mechanisms of resistance to aromatase inhibitors. Endocr Relat Cancer 2015;22:645-56. [Crossref] [PubMed]
- Thompson HJ, Sedlacek SM, Paul D, et al. Effect of dietary patterns differing in carbohydrate and fat content on blood lipidand glucose profiles based on weight-loss success of breast-cancer survivors. Breast Cancer Res 2012;14:R1. [Crossref] [PubMed]
- Wulaningsih W, Vahdaninia M, Rowley M, et al. Prediagnostic serum glucose and lipids in relation to survival in breast cancer patients: a competing risk analysis. BMC Cancer 2015;15:913. [Crossref] [PubMed]
- Rangasamy S, McGuire PG, Das A. Diabetic retinopathy and inflammation: novel therapeutic targets. Middle East Afr J Ophthalmol 2012;19:52. [Crossref] [PubMed]
- Stone PJ, Goodheart MJ, Rose SL, et al. The influence of microvessel density on ovarian carcinogenesis. Gynecol Oncol 2003;90:566-71. [Crossref] [PubMed]
- Van Poznak C, Somerfield MR, Bast RC, et al. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2015;33:2695-704. [Crossref] [PubMed]


