
The tumor-draining lymph nodes are immunosuppressed in patients with hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC), which accounts approximately half of the total of deaths occur in patients from China, is a malignant tumor associated with a poor prognosis (1). Hepatectomy remains the most effective therapy for HCC (2). Unfortunately, the rates of postsurgical recurrence and metastasis in 5 years was 50–70% (3). A growing amount of evidence suggests that the immune status of HCC was suppressed, for the high expression of PD-1 and TIM3 in tumor infiltrating T cells (4), which mediated suppression of tumor-infiltrating CD4+ or CD8+ T cells in HCC (5,6). This immunosuppressed status, which is conducive to tumor growth and recurrence, is one reason for the high recurrence rate of HCC (7-9). However, the mechanism of immunosuppressed state of HCC was still unknown.
The tumor-draining lymph nodes (TDLN) were the first location in which the specific recognition of tumor antigens and appropriate activation of lymphocytes occur during the early stage of most cancers (10,11). Thus, the immune status of TDLN influenced the immune status of HCC microenvironment (12). Discovering the immune status of TDLN may help us to understand the mechanism of immunosuppressed state of HCC. In another hand, increased evidences showed that tumor could also secret soluble factors to induce the immune suppression of TDLN in other cancer (13). However, the immune status of TDLN in HCC had not been well studied.
Thus, in this study, we studied the phenotypic characteristics, proliferative capacity and cytotoxic activity of the immune cells isolated from the TDLN of patients with HCC.
Methods
Patients and the source of TDLN and peripheral blood mononuclear cell (PBMC)
For TDLN, the portal node was chosen, as it was the main lymph node station of the liver (14). The TDLN and preoperative PBMC were collected from HCC patients (n=16) who underwent hepatectomy at the Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center. Patients who had previously received chemotherapy or radiotherapy before surgery were excluded from the study. In addition, the samples of another three HCC patients were used for the proliferation and cytotoxicity assay. Table S1 shows the distribution of the clinical background characteristics of the patients. The clinical informed consent was obtained from all patients enrolled in this study, and approval was obtained from the Ethics Committee of the Sun Yat-sen University Cancer Center, ID: 5010-2007-043.
Isolation of TDLN and PBMC
PBMC were separated from the blood by density gradient centrifugation using Ficoll (Nycomed Pharma AS, Norway) at 800 ×g for 30 min (15). TDLN cells were isolated from the TDLN by grinding, filtering through a 200 microns mesh sieve, and collecting the cell suspension. Then the cells were counted and resuspended at the density of 1×106/mL.
Immunophenotyping
The phenotypic characteristics of the cells was analyzed using flow cytometry (Beckman Coulter, Gallios) after collecting at the density of 106/mL, washing with PBS, and incubating with antibodies. The monoclonal antibodies CD3-ECD, CD4-PC7, CD8-FITC, CD25-PE, CD56-PE, CD69-APC, anti-IL-17-APC, anti-IFN-γ-PE, and anti-PD1-APC were purchased from BD Biosciences (CA, USA). Anti-Foxp3-APC were purchased from eBioscience (San Diego, USA). For intracellular cytokine detection, cells were stimulated for 4 h with Leukocyte Activation Cocktail (BD Biosciences), stained with surface markers, fixed and permeabilized with IntraPre Reagent (Beckman Coulter), and then stained with anti-IL-17 and anti-IFN-γ antibodies. The cocktail includes phorbol 12-myristate 13-acetate, ionomycin and Brefeldin A. For Foxp3 analysis, the anti-Foxp3 Staining Set (eBioscience) was used according to the manufacturer’s instructions. The flow cytometry data was analyzed with Kaluza analysis software (Beckman Coulter).
Expansion of cells from TDLN and PBMC
1×106 /mL cells derived from TDLN or PBMC were cultured in RPMI-1640 containing 10% FBS at 37 °C with 100 ng/mL mouse anti-human CD3 monoclonal antibody (Peprotech) and 1,000 U/mL IL-2 (Peprotech) were added to each wells. Fresh complete medium and IL-2 supplementation (1,000 U/mL) was added every three days. The suspension cells were then harvested after incubation for about 7 days for cytotoxicity assay.
Proliferation assay
To assess the proliferative capacity of the immune cells, the cells extracted from the TDLN and PBMC were stained with 2 mm carboxyfluorescein diacetate succinimidyl ester (CFSE) for 10 min at 37 °C in serum-free RPMI-1640. Cells were washed twice in RPMI with 10% FBS and stimulated with 100 ng/mL mouse anti-human CD3 monoclonal antibody (Peprotech) and 1,000 U/mL IL-2 (Peprotech). On the fourth day, the proliferation of cells was assayed via flow cytometry (Beckman Coulter, Gallios) by detecting the level of CFSE.
Tumor cell culture
The SMMC-7721 human HCC cell lines were obtained from the Cell Bank of the Chinese Academy of Science (Beijing, China). Cells were maintained in a 37 °C humidified incubator with 5% CO2, and cultured in RPMI1640 medium containing 10% FBS.
Cytotoxicity analysis
To detect the level of cytotoxicity, 1×105/mL cells from SMMC-7721 human HCC cell line were cultured in the absence (control) or with 1×106/mL cells expensed from TDLN or PBMC in six-well plates in 2 mL RPMI-1640 medium containing 10% FBS. After culturing the cells for 1 day, the SMMC-7721 cells were digested with 0.25% Trypsin-EDTA (Gibco, USA), washed, and stained with the Annexin V-PI apoptosis detection kit (BD Biosciences) according to the manufacturer’s instructions to detect the level of apoptosis. The cells were assessed using flow cytometer (Beckman Coulter, Gallios) and the data was analyzed with Kaluza analysis software (Beckman Coulter).
Statistical analyses
Continuous variables were expressed as the mean ± standard deviation (SD). Comparisons between the two groups were performed using a paired t-test or student’s t-test. All analyses were performed using IBM SPSS Statistics software, Version 20.0 (SPSS, Inc., IL, USA). P values less than 0.05 in the two-tailed test were considered to be significant.
Results
Phenotype of the T cells in TDLN and PBMC
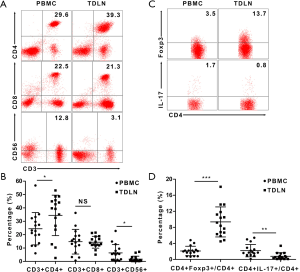
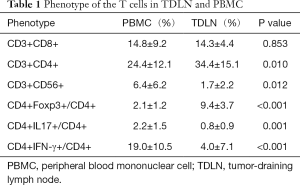
To study the immune status of TDLN, we used flow cytometry to compare the phenotypes of the cells separated from the TDLN and PBMC. The proportion of CD3+CD4+ T cells was higher in the TDLN than in the PBMC [34.4%±15.1% vs. 24.4%±12.1% (P=0.010)]. The proportion of CD3+C56+ T (NKT) cells was lower in the TDLN than the PBMC [1.7%±2.2% vs. 6.4%±6.2% (P=0.012)]. There were no differences in the proportion of CD3+CD8+ T cells between the TDLN and PBMC (Figure 1A,B) (Table 1).
Full table
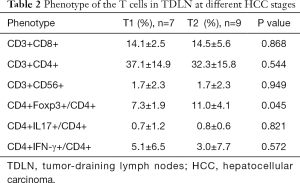
Subsequently, we compared the proportions of different CD4+ T cell subsets, including CD4+Foxp3+ T (Treg) cells and IL-17-producing CD4+ T (Th17) cells. The proportion of Treg/CD4+ cells was higher in the TDLN than in the PBMC [9.4%±3.7% vs. 2.1%±1.2% (P<0.001)] (Figure 1C), while the Th17/CD4+ cells was lower in the TDLN than in the PBMC [0.8%±0.9% vs. 2.2%±1.5% (P=0.001)] (Figure 1C,D) (Table 1). Moreover, we found that the proportion of Treg/CD4+ cells was lower in T1 stage than T2 stage tumors [7.3%±1.9% vs. 11.0%±4.1%, respectively (P=0.045)] (Table 2).
Full table
Together, these data suggest that the immune status of TDLN was suppressed and deteriorated by tumor progression.
Early activation of the T cells and capacity of the T cells in TDLN and PBMC
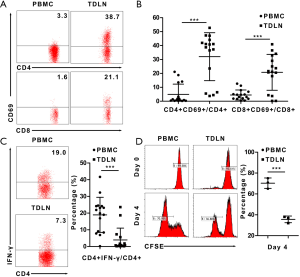
As the TDLN was the first location in which the specific recognition of tumor antigens and appropriate activation of lymphocytes occur, we want to know whether the T cells presented early activation. As CD69 was considered as a marker of early activation of T cells (16), we detected the expression of CD69 by using flow cytometry and found that both CD4+ and CD8+ T cells subgroup were more activated in the TDLN than the PBMC. The proportion of CD4+CD69+/CD4+ cells in the TDLN was 32.0%±17.2% vs. 5.0%±7.3% in the PBMC (P<0.001); and CD8+CD69+/CD8+ cells in the TDLN was 20.8%±12.9% vs. 4.5%±3.6% in the PBMC (P<0.001) (Figure 2A,B). In another hand, considering that the immune status of TDLN was suppressed described above; we explored capacity of T cells. First, we explored the proportions of IFN-γ-producing CD4+ T (Th1) cells, which lead to cell mediated anti-tumor immunity. We found that the proportions of Th1/CD4+ cells were lower in the TDLN than in the PBMC [4.0%±7.1% vs. 19.0%±10.5% (P<0.001)] (Figure 2C). Next, we measured the level of CFSE dilution exhibited by CFSE-labeled cells from the TDLN and PBMC, to explore the proliferative capability of the T cells. The mean proliferation rate of T cells in the TDLN was lower (35.6%±3.2%) compared to the PBMC (70.3%±5.0%) (P=0.001) (Figure 2D). Together, these data suggest that the T cells were activated, while the capacity was suppressed.
PD-1 expression of the T cells in TDLN and PBMC
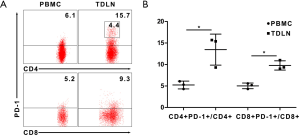
To further discover the discordance between immunosuppressed status and increased activated T cells, we investigate the expression of PD-1 in T cells. We found that the proportion in each T cells subgroup was higher in the TDLN than the PBMC. The proportion of CD4+PD-1+/CD4+ cells in the TDLN was 13.5%±3.6% vs. 5.2%±0.9% in the PBMC (P=0.037); and CD8+PD-1+/CD8+ cells in the TDLN was 9.8%±1.1% vs. 5.0%±0.7% in the PBMC (P=0.042) (Figure 3A,B). In TDLN, a population of PD-1 high CD4+ T cells (3.2%±1.9%) could be discerned that was absent in PBMC (Figure 3A). However, PD-1 high population was not apparent in the CD8+ T cells. Together, these data suggest that the high expression of PD-1 may suppress the function of T cells.
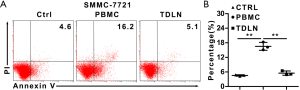
Cytotoxicity of the cells expanded from TDLN and PBMC
Last, we want to explore the cytotoxicity of the immune cells derived from the TDLN in vitro. We performed co-culture experiments in which the cells expended from TDLN or PBMC were cultured with SMMC-7721 cells for 1 day. The apoptosis ratio of the SMMC-7721 cells was higher in the PBMC group compared to the TDLN group (16.5%±1.6% and 5.5%±0.9%) (P=0.002) (Figure 4A,B). Together, these data suggest that the anti-tumor capacity of immune cells in TDLN was suppressed.
Discussion
The findings of the present study demonstrated the immunosuppressed state of the TDLN in patients with HCC, characterized by a higher proportion of Tregs, lower proportion of NKT cells, Th17 and Th1 cells. The T cells from TDLN exhibited decreased capacity of proliferation with higher expression of PD-1 compared to PBMC. And the cytotoxic activity of the cells expanded from the TDLN was weaker than the PBMC
Although the TDLN is the primary location for the generation of the anti-tumor immune response, the immune status of the TDLN is suppressed in various cancers (17,18). Our data also found that the proportion of CD3+C56+ NKT cells was lower in the TDLN than the PBMC. Recent studies have found that the accumulation of Tregs, playing an important role in maintaining immune homeostasis and preventing chronic inflammatory diseases (19), in the TDLN in various cancers (18,20-23) may contribute to the immunosuppressed state of this region. In accordance with these studies, we also identified a higher proportion of Treg in the TDLN compared to the PBMC. Moreover, the proportion of Tregs in the TDLN increased by tumor progression, which was similar to other study (24). Various studies have partially uncovered the mechanisms by which Tregs exert their immunosuppressive function (19). These methods of immunosuppression include the secretion of soluble inhibitory molecules, the induction of cytolysis of target cells, expression of membrane tethered inhibitory molecules, and the inhibition of dendritic cell (DCs) maturation (25). For example, IL-10, IL-35, and TGF-β are considered to be suppressive cytokines and are secreted by Tregs (25). Moreover, by Granzyme B and Perforin, Treg could make the cytolysis of NK cells (25). This partially explains the higher proportion of Tregs and lower number of NKT cells (CD3+CD56+) in the TDLN compared to the PBMC in our study. In addition, glucocorticoid-induced TNF-receptor family related protein (GITR) expression on Tregs has become an attractive target for cancer immunotherapy. Thus, the administration of an agonistic anti-GITR antibody was found to block the suppressive effects of Tregs and increase IFN-γ-producing CD8+ and CD4+ T cell infiltration (26,27). This observation is in accordance with our findings, in which the CD4+ T cell population from the TDLN produced lower levels of IFN-γ. The consistency between our study and previous reports confirms the pivotal role of Tregs in the immunosuppressive environment of the TDLN.
The TDLN was the first location where antigen-presenting cells interact with T lymphocytes and initiate immune responses to cancer (28). Our results also showed that the proportion of activated T cells (CD4+CD69+ T cells, and CD8+CD69+ T cells) was higher in the TDLN compared to the PBMC. Takenoyama et al. (29) also found that the cells derived from the TDLN in patients with primary lung cancer were activated to a greater extent than PBMC by detecting activation-related molecules. Recent research has also demonstrated that there was an enrichment of mature DCs in the TDLN, which plays an important role in supporting the activation of T cells (30). Through effective interactions with tumor antigens loaded by DCs in the TDLN, there may be an increased generation of activated T cells in this region; however, as described above, our data revealed that the capacity of cellular proliferation were lower in the TDLN compared to the PBMC in patients with HCC. Moreover, the proportions of IFN-γ-producing CD4+ T (Th1) cells were lower in the TDLN than in the PBMC. These data gave us a hint that the T cells were activated, while the capacity was suppressed.
Thus, to further discovery the discordance between immunosuppressed status and increased activated T cells, we investigate the expression of PD-1 in T cells, which could inhibit T cells and block the antitumor immune response (31). In addition, we found that the T cells expressed higher PD-1, especially CD4+ T cells, in TDLN than PBMC. Recent studies showed that PD-1 could suppress specific CD8+ T cell cytotoxicity via suppress cytokine production in tumor and TDLN (32,33). In accordance with these studies we also found that the expanded T cells from TDLN presented a low level of cytotoxicity and this may associate with high expression of PD-1. These data indicated that even though the tumor antigen could increase the number of activated T cells, the higher expression of PD-1 in T cells, especially CD4+ T cell, may suppress the capacity of CD4+ and CD8+ T cell. Together, these data gave us a hint that anti-PD-1 treatment may retrieve the immunosuppressed status of TDLN and induce antitumor immune response, which need to be further studied.
In the present study, we showed that the immune cells from TDLN exhibit an immunosuppressed status reflected in the phenotypic characteristics, as well as a lower proliferative, cytotoxic activity and higher expression of PD-1 compared to the PBMC. Moreover, the immune status of the TDLN was further suppressed with tumor progression. Our results suggest that the immune status of HCC microenvironment was suppressed and this immunosuppressed state may be reversed by inhibiting Treg cells and/or PD-1. Further research is required to determine if this strategy will favor the overall survival of patients with HCC.
Conclusions
In conclusion, according to our study, the immune cells from the TDLN presented an immunosuppressed status regarding both the phenotypic characteristics and cellular immune function compared to PBMC.

Full table
Acknowledgments
We would like to thank Elixigen Company for English language editing.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.11.14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The clinical informed consent was obtained from all patients enrolled in this study, and approval was obtained from the Ethics Committee of the Sun Yat-sen University Cancer Center, ID: 5010-2007-043.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Lee CW, Kuo WL, Yu MC, et al. The expression of cytokeratin 19 in lymph nodes was a poor prognostic factor for hepatocellular carcinoma after hepatic resection. World J Surg Oncol 2013;11:136. [Crossref] [PubMed]
- Gish RG, Porta C, Lazar L, et al. Phase III randomized controlled trial comparing the survival of patients with unresectable hepatocellular carcinoma treated with nolatrexed or doxorubicin. J Clin Oncol 2007;25:3069-75. [Crossref] [PubMed]
- Zhou G, Sprengers D, Boor PPC, et al. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology 2017;153:1107-19 e10.
- Kuang DM, Zhao Q, Peng C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 2009;206:1327-37. [Crossref] [PubMed]
- Li H, Wu K, Tao K, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 2012;56:1342-51. [Crossref] [PubMed]
- Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009;15:971-9. [Crossref] [PubMed]
- Brittenden J, Heys SD, Ross J, et al. Natural killer cells and cancer. Cancer 1996;77:1226-43. [Crossref] [PubMed]
- Taketomi A, Shimada M, Shirabe K, et al. Natural killer cell activity in patients with hepatocellular carcinoma: a new prognostic indicator after hepatectomy. Cancer 1998;83:58-63. [Crossref] [PubMed]
- Santin AD. Lymph node metastases: the importance of the microenvironment. Cancer 2000;88:175-9. [Crossref] [PubMed]
- Evans EM, Man S, Evans AS, et al. Infiltration of cervical cancer tissue with human papillomavirus-specific cytotoxic T-lymphocytes. Cancer Res 1997;57:2943-50. [PubMed]
- Munn DH, Mellor AL. The tumor-draining lymph node as an immune-privileged site. Immunol Rev 2006;213:146-58. [Crossref] [PubMed]
- Tempesta E, Janiri L, Pirrongelli C. Stereospecific effects of acetylcarnitine on the spontaneous activity of brainstem neurones and their responses to acetylcholine and serotonin. Neuropharmacology 1985;24:43-50. [Crossref] [PubMed]
- Barbier L, Tay SS, McGuffog C, et al. Two lymph nodes draining the mouse liver are the preferential site of DC migration and T cell activation. J Hepatol 2012;57:352-8. [Crossref] [PubMed]
- Kuang DM, Wu Y, Chen N, et al. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood 2007;110:587-95. [Crossref] [PubMed]
- Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol 2005;26:136-40. [Crossref] [PubMed]
- Munn DH, Sharma MD, Hou D, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest 2004;114:280-90. [Crossref] [PubMed]
- Gai XD, Song Y, Li C, et al. Potential role of plasmacytoid dendritic cells for FOXP3+ regulatory T cell development in human colorectal cancer and tumor draining lymph node. Pathol Res Pract 2013;209:774-8. [Crossref] [PubMed]
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008;8:523-32. [Crossref] [PubMed]
- Viguier M, Lemaitre F, Verola O, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol 2004;173:1444-53. [Crossref] [PubMed]
- Tien AH, Xu L, Helgason CD. Altered immunity accompanies disease progression in a mouse model of prostate dysplasia. Cancer Res 2005;65:2947-55. [Crossref] [PubMed]
- Peng L, Kjaergaard J, Plautz GE, et al. Tumor-induced L-selectinhigh suppressor T cells mediate potent effector T cell blockade and cause failure of otherwise curative adoptive immunotherapy. J Immunol 2002;169:4811-21. [Crossref] [PubMed]
- Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 2002;169:2756-61. [Crossref] [PubMed]
- Deng L, Zhang H, Luan Y, et al. Accumulation of foxp3+ T regulatory cells in draining lymph nodes correlates with disease progression and immune suppression in colorectal cancer patients. Clin Cancer Res 2010;16:4105-12. [Crossref] [PubMed]
- Liu C, Workman CJ, Vignali DA. Targeting Regulatory T Cells in Tumors. FEBS J 2016;283:2731-48. [Crossref] [PubMed]
- Ko K, Yamazaki S, Nakamura K, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med 2005;202:885-91. [Crossref] [PubMed]
- Shimizu J, Yamazaki S, Takahashi T, et al. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol 2002;3:135-42. [Crossref] [PubMed]
- Zheng R, Shu S. Immune response to cancer and its regulation in regional lymph nodes. J Surg Oncol 2011;103:550-4. [Crossref] [PubMed]
- Takenoyama M, Yasumoto K, Harada M, et al. Antitumor response of regional lymph node lymphocytes in human lung cancer. Cancer Immunol Immunother 1998;47:213-20. [Crossref] [PubMed]
- Kimura H, Dobrenkov K, Iida T, et al. Tumor-draining lymph nodes of primary lung cancer patients: a potent source of tumor-specific killer cells and dendritic cells. Anticancer Res 2005;25:85-94. [PubMed]
- Errico A. Immunotherapy: PD-1-PD-L1 axis: efficient checkpoint blockade against cancer. Nat Rev Clin Oncol 2015;12:63. [Crossref] [PubMed]
- Maier H, Isogawa M, Freeman GJ, et al. PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol 2007;178:2714-20. [Crossref] [PubMed]
- Wu X, Zhang H, Xing Q, et al. PD-1(+) CD8(+) T cells are exhausted in tumours and functional in draining lymph nodes of colorectal cancer patients. Br J Cancer 2014;111:1391-9. [Crossref] [PubMed]


