
Circulating cell free deoxyribonucleic acid for tracking early treatment response and disease progression in advanced cancers
Introduction
Analysis of circulating cell free deoxyribonucleic acid (cfDNA) is a promising and emerging strategy in oncology research because of its potential to improve diagnosis and facilitate precision medicine. A favourable characteristic of cfDNA with respect to cancer patients is that it incorporates circulating tumour DNA (ctDNA) and hence provides insight into tumour biology and extent of disease from plasma or serum samples. The present review provides a perspective on the current literature pertaining to the use of ctDNA in predicting early treatment response and disease progression, as well as tracking disease progression in advanced cancers.
cfDNA
DNA, which is typically contained within the nucleus, can exit the cell and appear in extracellular fluids such as blood and lymph by a variety of cell processes (1), in particular by cell death occurring through either apoptosis or necrosis. The extracellular DNA that is found in bodily fluid is typically present as small fragments bound to proteins such as histones and is described as cfDNA.
Cell death occurring through either apoptosis or necrosis releases DNA fragments into the systemic circulation in all individuals. The overall level of cfDNA is a dynamic balance between the processes of cfDNA release and the mechanisms of DNA degradation and clearance. In healthy individuals, the cfDNA is rapidly cleared by various mechanisms such as degradation by blood nucleases (2) and uptake and degradation by phagocytes thereby keeping cfDNA levels low (1,3,4). However, certain conditions such as inflammation may increase DNA release into blood by either increasing the rate of cell death or reducing the clearance of cell debris (1,3,5).
Previous research has explored cfDNA in conditions such as diabetes, stroke, systemic lupus erythematosus, trauma and rheumatoid arthritis (6-10). However, the majority of cfDNA based work in medicine is still in an early research phase (11). Notably, prenatal genetic testing based on cell free foetal DNA (cffDNA) has become available since 2011 and has changed the landscape of prenatal aneuploidies testing. The test is highly sensitive and specific for most common aneuploidies and has reduced the need for sampling of foetal genetic material through more invasive techniques such as chorionic villus sampling or amniocentesis that pose a small but very significant risk of foetal loss (11-13).
cfDNA has attracted particular interest as a potential blood biomarker in cancer, often referred to as a ‘liquid biopsy’. Changes in cfDNA levels of cancer patients are influenced by cancer related factors such as type of cancer, stage, grade, location and size (14-16). Additionally, cfDNA allows the possibility of longitudinal tracking of the responses to treatment.
ctDNA
Tumour cell DNA may differ from germline cell DNA as tumour cells undergo genetic alterations that include oncogene and tumour suppressor gene mutations, hypermethylation and microsatellite alterations (14,16,17). The DNA released by tumour cells contains these tumour specific genetic alterations and thus a proportion of cfDNA found in biological fluids of a cancer patient contain tumour specific mutated fragments that are called circulating cell free ctDNA. Apart from the primary tumour tissue, tumour cells that are circulating in the blood and metastatic deposits present at distant sites also release ctDNA (3-5).
ctDNA often constitutes a very small proportion of cfDNA, being as low as 0.005% (18) and hence highly sensitive detection methods is a prerequisite for ctDNA based applications. Recent advances in sequencing technologies have led to the development of highly sensitive and specific methods to detect ctDNA at frequencies as low as 0.001% (19,20). The major focus of ctDNA application to date has been on identifying tumour mutations that guide treatment selection. This may eventually enable surgical tumour biopsies to be avoided and is particularly important for cancers where tumour biopsies are difficult to obtain such as lung cancer. Two ctDNA based diagnostic tests have already been approved by FDA for EGFR mutation testing for treatment selection in patients with non-small-cell lung cancer (21).
More recently, the value of ctDNA as a biomarker is being explored for a range of distinct clinical applications such as cancer screening, confirming diagnosis, tracking treatment response and tracking disease progression. This review specifically focuses on the validity and utility of cfDNA and ctDNA in predicting early treatment response and in tracking disease progression in advanced cancers.
Monitoring treatment response to cancer medicines
Treatment monitoring is an essential part of clinical management that helps to establish therapeutic effectiveness. Treatment response to cancer medicines is often monitored by physical examination, serial radiological imaging and in selected cancers by circulating tumour markers (22-25).
Response evaluation criteria in solid tumour (RECIST), which is based on radiological imaging, is the current gold standard for monitoring treatment response in the setting of advanced cancer. The RECIST guidelines categorises treatment response as complete response (CR; undetectable tumour), partial response (PR; >30% decrease in target tumour size), progressive disease (PD; >20% increase in target tumour size) or stable disease (SD; neither sufficient tumour shrinkage to qualify for PR nor sufficient increase to qualify for PD) (26). Radiological imaging is generally performed every 2–6 months depending on the type of cancer (22,24) and hence there is an opportunity to track response more frequently. Additionally, radiological imaging may be limited by insensitivity to small lesions (<10 mm), inter-scorer variability, the significant costs and patient exposure to potentially harmful radiation.
For a limited number of cancers circulating blood markers are currently used to track treatment response. This includes prostate specific antigen (PSA) for prostate cancer, carcinoembryonic antigen (CEA) and cancer antigen 15-3 (CA 15-3) for breast cancer. These biomarkers are not generalizable to all cancer types and often lack sufficient sensitivity and specificity to monitor treatment response in isolation (27). For example, CA 15-3 and CEA are only recommended to be used in conjunction with diagnostic imaging, medical history and physical examination for treatment monitoring and decision making (27,28).
There are two major aspects of monitoring treatment response to cancer medicines. Firstly, tracking initial response to treatment, and secondly, tracking loss of response and the resulting progression of the disease. The use of ctDNA to track both initial response and disease progression for advanced cancers presents a significant opportunity. To date there are no reviews that describe usefulness of cfDNA and ctDNA in predicting early treatment response and in tracking early disease progression across a range of advanced cancers.
Literature search
Studies were searched on PubMed, Scopus, Google Scholar, and Google using the keywords [‘cell free DNA’, ‘circulating DNA’, ‘circulating cell free DNA’, ‘cfDNA’, ‘ctDNA’, ‘cell free tumour DNA’ or ‘ctDNA’] and [‘treatment response’ or ‘early response’] and [‘metastatic cancer’, ‘metastatic disease’ or ‘advanced cancer’]. The search results were screened by the title of the study followed by the abstract. The selection criteria included the studies that have collected at least one blood sample after treatment initiation and have reported treatment response and/or disease progression. In total, there were 16 original studies that were identified as relevant and were considered for this review.
Early on-treatment prediction of treatment response
CfDNA approach
Two small studies have reported promising preliminary results suggesting cfDNA may predict treatment response as early as 4 weeks after commencing treatment in advanced cancers (Table 1). Early in chemotherapy treatment (week 4 and 8) for non-small cell lung cancer (NSCLC) patients (n=42), the cfDNA levels were significantly lower for patients who would eventually respond (best overall response of PR or CR by imaging) compared to patients who would not respond (29). Similarly, renal cell carcinoma patients using sorafenib (n=18) that had radiologically confirmed disease control (partial response or stable disease) at week 12 were found to have decreased cfDNA levels at week 8. In contrast, patients with progressive disease had increase in cfDNA levels at week 8 (30).

Full table
These results indicate that quantitative changes in total cfDNA levels during early treatment can potentially predict treatment response. While, the results are encouraging; there are distinct differences observed in the studies. Kumar et al. showed decrease in cfDNA in all the patients following treatment with the degree of cfDNA reduction being the discriminator between responders and non-responders. In contrast, Feng and colleagues observed an increased cfDNA in some individuals and grouped patients on the basis of increase or decrease in cfDNA (29,30). This indicates that cfDNA trends and cut points may vary between types of cancers and treatments. Further studies are required across a range of cancers and treatment types before any common patterns can be identified. Future studies should employ sufficiently large number of patients to validate the preliminary results reported in these two studies. The reporting results also needs to be standardised. For example, Feng et al. has not reported the sensitivity and specificity of predicting early response making it difficult to compare results (30).
Circulating tumour or hypermethylated cfDNA approaches
Eight studies have reported the evaluation of ctDNA or hypermethylated cfDNA (mcfDNA) as an early marker of treatment response in advanced colorectal cancer, lung cancer and melanoma (Table 2). Two different approaches to analysing and reporting the association between ctDNA and response were used. The first approach was based on the relative ctDNA levels between baseline and the post-baseline time point, and commonly a 2-fold reduction in ctDNA was used as the cut-point. The second approach was to group ctDNA levels in 3 different categories based on patterns baseline and post-baseline levels—for example, undetectable ctDNA levels at baseline & during therapy, detectable at baseline but undetectable during early therapy or detectable ctDNA at baseline and during therapy (35). In addition, one study used detectable or undetectable level of mcfDNA as a biomarker of response. Most of these studies were relatively small (n<100), with the exception of the study of hypermethylation.
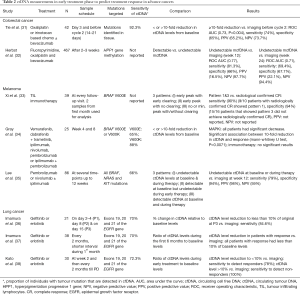
Full table
Both methods of ctDNA change indicated that early changes in ctDNA levels can be predictive of treatment response (Table 2). For example, a 10-fold ctDNA decrease following treatment predicted the treatment response with PPV of 65.2% and NPV of 73.7% at 2–3 weeks post-treatment for patients with advanced colorectal cancer (31). Patterns of ctDNA changes also predicted early treatment response with reasonably high sensitivity and specificity. In addition, hypermethylation approach was highly specific (NPV at week 2–3=98&94 for week 12 and 24, respectively) at predicting early treatment response (32).
There is a considerable amount of work needed to standardise the use of ctDNA based ‘liquid biopsies’ in clinical practice. The studies performed so far have employed distinct methods thereby making it difficult to compare and aggregate the findings. For example, some studies have utilised absolute copy numbers of ctDNA whereas others have utilised relative ctDNA levels (i.e., as a fraction of total cfDNA) (35,38). Given the high inter and intra-patient variabilities in ctDNA levels, it may be more appropriate to undertake a head-to-head comparison of methods to evaluate the best approach for ctDNA analysis and reporting.
The blood sampling schedules were also variable across the studies. Some studies had relatively intensive sampling schedules, collecting multiple samples in first 2 weeks of the treatment while others did not collect any samples until week 4–8 (31,34,36). Intensive sampling during the first few weeks of the treatment is generally the preferred approach as it enables response prediction at very early stage in the treatment. Harmonising sample collection schedules across studies of the same cancer and treatment type will facilitate the comparison and aggregation of results between studies. In addition, studies employed different statistical approaches to assess and report associations. Some studies reported sensitivity, specificity, PPV and NPV of ctDNA levels in predicting early treatment response whereas others only reported the statistical significance of the association between ctDNA results and radiological imaging (34,36-38). A broad consensus should be formed around reporting the results of pre-specified parameters that generally should include the sensitivity and specificity of ctDNA approaches.
Tracking disease progression and/or treatment resistance
Nine studies have reported on the use of ctDNA to track disease progression or treatment resistance. Two different approaches or a combination of thereof were used to monitor disease progression. The first approach comprises quantitatively monitoring the ctDNA levels of the known baseline tumour mutations over a period of time to identify increases in ctDNA levels corresponding the disease progression. The other approach encompasses monitoring for the appearance or amplification of secondary/acquired mutations known to cause treatment resistance and/or disease progression. The first approach solely relies on quantitative changes in ctDNA levels whereas the second approach may include quantitative as well as qualitative changes in ctDNA.
Five studies reported on the quantification of ctDNA using mutations present at baseline to track disease progression for breast cancer, lung cancer and melanoma. Across these studies, elevated levels of ctDNA (baseline tumour mutations) was detected at time of PD (as determined by radiological imaging) for 50% to 90% of patients. ctDNA (baseline tumour mutations) elevations could be detected in advance of PD by radiological imaging for 55% to 65% of patients. In this subset of patients, the degree of lead time (time disease progression or treatment resistance is detected earlier than radiological assessment) was reported variably. In breast cancer a median lead time of this subset was reported to be 5 months (39), whereas a study of patients with advanced NSCLC treated with an EGFR inhibitor reported that progression could be detected at least 100 days prior to radiological imaging for 14% of patients (38).
Five studies reported on the evaluation of ctDNA based on acquired mutations to track resistance or progressive disease for advanced breast cancer, lung cancer and melanoma (Table 3). At the time progressive disease was detected by radiological imaging, the acquired mutation was detected in 30% to 85% of patients, depending on the study.
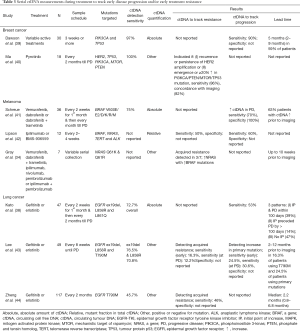
Full table
Notably, for two studies both existing and acquired mutations were tracked. In a small study of patients treated with immunotherapy for melanoma, the ability of existing and acquired mutations to track disease progression was similar (42). In a moderately sized study of patients with NSCLC treated with an EGFR inhibitor, acquired mutations were detected in approximately 29% of patients prior to or at the time of disease progression (43). In contrast, elevated levels of ctDNA based on an existing mutation were detected in about 55% of patients within the similar time frame.
The utility of the tracking ctDNA based on detection of acquired mutations is limited by its precondition of knowing the secondary or resistance causing mutations a priori. In many types of cancers, the likely acquired mutations are unknown and hence the approach may not be suitable. It may possibly be used in combination with the quantitative tracking of ctDNA based on known existing mutations and thereby possibly increase the overall sensitivity and concordance of ctDNA with radiological imaging (40). Further studies are required to evaluate the combination of these approaches.
The rule defining how progressive disease is identified base on changes in ctDNA levels differs between studies. Some studies have defined ctDNA based disease progression as an increase in ctDNA level at any time point following treatment response, whereas other studies have only considered it as a true disease progression if it has been detected within a certain time-period of radiologically diagnosed disease progression (38,39). Random fluctuations in their ctDNA levels will likely lead to the potential for false positives unless more sophisticated methods or rules are used (39). Future studies should evaluate a range of pre-defined rules for classifying disease progression based on ctDNA. For example, it is likely that rule based on a larger (e.g., 10-fold) or consistent ctDNA increase will reduce the risk of falsely calling disease progression.
Analytical and technical considerations
Various pre-analytical factors such as clotting, time to separate blood cells from plasma, freeze-thawing, isolation methods and storage may affect the integrity and total yield of cfDNA and subsequent ctDNA analysis (45-47). To date there has been significant pre-analytical variabilities across the conducted studies. For instance, the time to process blood cells from plasma varied from 3 to 24 hours after sample collection (31,34). The storage temperature of samples also varied from −20 to −80 °C (34,35). Additionally, the choice of using either plasma or serum for analysis may impact the results. The cfDNA concentration is 3–24 times higher in serum in comparison to plasma (48). However, cfDNA extracted from serum samples can possibly be extensively contaminated by the DNA released from immune cells during clotting (48). It is also more likely to show greater variations in cfDNA concentrations when a delay in storage occurs (48). On the other hand, cfDNA levels in plasma are low in comparison to serum but show less fluctuations to pre-analytical differences (48).
The sensitivity of ctDNA to detect the genetic alterations is quite varied across the studies. While, some studies have shown a remarkable rate of detecting the mutations in more than 90% of samples (31,39), the majority of the studies have had more modest detection rates of 65–70%. Tracking multiple mutations is one potential option for improving the proportion of patients with detectable ctDNA. The ongoing advances in the sequencing technology as well as development of new ultrasensitive detection methods may also contribute to improved ctDNA detection in the future (49,50).
Conclusions
The potential to utilise ctDNA detection in cancer treatment has emerged rapidly over the last 5 years. ctDNA has demonstrated preliminary but promising results as an early on-treatment predictor of treatment response and as a means of tracking disease progression/treatment resistance in advanced cancers. However, the current studies are relatively small in patient size and use variable approaches. Thus, it is important to replicate the findings in large independent studies with pre-specified analysis plans.
While, cfDNA displays greater inter-patient and inter-population variability than ctDNA, the approach is relatively less expensive to perform in comparison to ctDNA analysis. Other approaches that combine the use of cfDNA and ctDNA should also be considered in future work to potentially improve the predictive performance of cfDNA/ctDNA based liquid biopsies. The ctDNA approach needs further developmental work in terms of standardisation of ctDNA quantitative methods and techniques and harmonisation of methods for evaluating predictive performance and results reporting. Quantitative analysis of ctDNA and using ctDNA to detect secondary mutations are two emerging methods to track disease progression and treatment resistance. However, both methods, singularly or in combination, require further research to improve the sensitivity and specificity as well as the concordance of the ctDNA results with radiological assessments. Future work needs to focus on evaluating the best method to define ctDNA based disease progression in different cancer types and treatments. Ongoing improvements in analytical techniques to detect and quantify ctDNA will increase the sensitivity of ctDNA to detect mutations at very low levels and improve the performance characteristics of ctDNA.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Precision dosing of targeted anticancer drugs”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.11.21). The series “Precision dosing of targeted anticancer drugs” was commissioned by the editorial office without any funding or sponsorship. AR and MJS served as the unpaid Guest Editors of the series and serves as the unpaid editorial board members of Translational Cancer Research. The authors have no other conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pisetsky DS, Fairhurst AM. The origin of extracellular DNA during the clearance of dead and dying cells. Autoimmunity 2007;40:281-4. [Crossref] [PubMed]
- Tamkovich SN, Cherepanova AV, Kolesnikova EV, et al. Circulating DNA and DNase activity in human blood. Ann N Y Acad Sci 2006;1075:191-6. [Crossref] [PubMed]
- Crowley E, Di Nicolantonio F, Loupakis F, et al. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol 2013;10:472-84. [Crossref] [PubMed]
- Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011;11:426-37. [Crossref] [PubMed]
- Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov 2016;6:479-91. [Crossref] [PubMed]
- Leon SA, Shapiro B, Sklaroff DM, et al. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res 1977;37:646-50. [PubMed]
- Shapiro B, Chakrabarty M, Cohn EM, et al. Determination of circulating DNA levels in patients with benign or malignant gastrointestinal disease. Cancer 1983;51:2116-20. [Crossref] [PubMed]
- Lo YM, Rainer TH, Chan LY, et al. Plasma DNA as a prognostic marker in trauma patients. Clin Chem 2000;46:319-23. [PubMed]
- Koffler D, Agnello V, Winchester R, et al. The Occurrence of Single-Stranded DNA in the Serum of Patients with Systemic Lupus Erythematosus and Other Diseases. J Clin Invest 1973;52:198-204. [Crossref] [PubMed]
- Amoura Z, Piette JC, Chabre H, et al. Circulating plasma levels of nucleosomes in patients with systemic lupus erythematosus: correlation with serum antinucleosome antibody titers and absence of clear association with disease activity. Arthritis Rheum 1997;40:2217-25. [Crossref] [PubMed]
- Gahan PB. Circulating nucleic acids in early diagnosis, prognosis and treatment monitoring an introduction. advances in predictive, preventive and personalised medicine, Vol 5. London: Springer, 2015.
- Valderramos SG, Rao RR, Scibetta EW, et al. Cell-free DNA screening in clinical practice: abnormal autosomal aneuploidy and microdeletion results. Am J Obstet Gynecol 2016;215:626.e1-626.e10. [Crossref] [PubMed]
- Platt LD, Janicki MB, Prosen T, et al. Impact of noninvasive prenatal testing in regionally dispersed medical centers in the United States. Am J Obstet Gynecol 2014;211:368.e1-7. [Crossref] [PubMed]
- Jung K, Fleischhacker M, Rabien A. Cell-free DNA in the blood as a solid tumor biomarker--a critical appraisal of the literature. Clin Chim Acta 2010;411:1611-24. [Crossref] [PubMed]
- Schwarzenbach H, Pantel K. Circulating DNA as biomarker in breast cancer. Breast Cancer Res 2015;17:136. [Crossref] [PubMed]
- Board RE, Knight L, Greystoke A, et al. DNA Methylation in Circulating Tumour DNA as a Biomarker for Cancer. Biomark Insights 2008;2:307-19. [PubMed]
- Openshaw MR, Page K, Fernandez-Garcia D, et al. The role of ctDNA detection and the potential of the liquid biopsy for breast cancer monitoring. Expert Rev Mol Diagn 2016;16:751-5. [Crossref] [PubMed]
- Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med 2008;14:985-90. [Crossref] [PubMed]
- Leary RJ, Sausen M, Kinde I, et al. Detection of chromosomal alterations in the circulation of cancer patients with whole-genome sequencing. Sci Transl Med 2012;4:162ra154 [Crossref] [PubMed]
- Bardelli A, Corso S, Bertotti A, et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov 2013;3:658-73. [Crossref] [PubMed]
- cobas EGFR Mutation Test v2. Available online: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm504540.htm
- Graham LJ, Shupe MP, Schneble EJ, et al. Current approaches and challenges in monitoring treatment responses in breast cancer. J Cancer 2014;5:58-68. [Crossref] [PubMed]
- Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1-v27. [Crossref] [PubMed]
- Trotter SC, Sroa N, Winkelmann RR, et al. A global review of melanoma follow-up guidelines. J Clin Aesthet Dermatol 2013;6:18-26. [PubMed]
- Walker AS, Zwintscher NP, Johnson EK, et al. Future directions for monitoring treatment response in colorectal cancer. J Cancer 2014;5:44-57. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Harris L, Fritsche H, Mennel R, et al. American society of clinical oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 2007;25:5287-312. [Crossref] [PubMed]
- Bast RC Jr, Ravdin P, Hayes DF, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American society of clinical oncology. J Clin Oncol 2001;19:1865-78. [Crossref] [PubMed]
- Kumar S, Guleria R, Singh V, et al. Plasma DNA level in predicting therapeutic efficacy in advanced nonsmall cell lung cancer. Eur Respir J 2010;36:885-92. [Crossref] [PubMed]
- Feng G, Ye X, Fang F, et al. Quantification of plasma cell-free DNA1 in predicting therapeutic efficacy of sorafenib on metastatic clear cell renal cell carcinoma. Dis Markers 2013;34:105-11. [Crossref] [PubMed]
- Tie J, Kinde I, Wang Y, et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol 2015;26:1715-22. [Crossref] [PubMed]
- Herbst A, Vdovin N, Gacesa S, et al. Methylated free-circulating HPP1 DNA is an early response marker in patients with metastatic colorectal cancer. Int J Cancer 2017;140:2134-44. [Crossref] [PubMed]
- Xi L, Pham TH, Payabyab EC, et al. Circulating tumor DNA as an early indicator of response to t-cell transfer immunotherapy in metastatic melanoma. Clin Cancer Res 2016;22:5480-6. [Crossref] [PubMed]
- Gray ES, Rizos H, Reid AL, et al. Circulating tumor DNA to monitor treatment response and detect acquired resistance in patients with metastatic melanoma. Oncotarget 2015;6:42008-18. [Crossref] [PubMed]
- Lee JH, Long GV, Boyd S, et al. Circulating tumour DNA predicts response to anti-PD1 antibodies in metastatic melanoma. Ann Oncol 2017;28:1130-6. [Crossref] [PubMed]
- Imamura F, Uchida J, Kukita Y, et al. Early responses of EGFR circulating tumor DNA to EGFR tyrosine kinase inhibitors in lung cancer treatment. Oncotarget 2016;7:71782-9. [PubMed]
- Imamura F, Uchida J, Kukita Y, et al. Monitoring of treatment responses and clonal evolution of tumor cells by circulating tumor DNA of heterogeneous mutant EGFR genes in lung cancer. Lung Cancer 2016;94:68-73. [Crossref] [PubMed]
- Kato K, Uchida J, Kukita Y, et al. Numerical indices based on circulating tumor DNA for the evaluation of therapeutic response and disease progression in lung cancer patients. Sci Rep 2016;6:29093. [Crossref] [PubMed]
- Dawson SJ, Tsui DW, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 2013;368:1199-209. [Crossref] [PubMed]
- Ma F, Zhu W, Guan Y, et al. ctDNA dynamics: a novel indicator to track resistance in metastatic breast cancer treated with anti-HER2 therapy. Oncotarget 2016;7:66020-31. [PubMed]
- Schreuer M, Meersseman G, Van Den Herrewegen S, et al. Quantitative assessment of BRAF V600 mutant circulating cell-free tumor DNA as a tool for therapeutic monitoring in metastatic melanoma patients treated with BRAF/MEK inhibitors. J Transl Med 2016;14:95-105. [Crossref] [PubMed]
- Lipson EJ, Velculescu VE, Pritchard TS, et al. Circulating tumor DNA analysis as a real-time method for monitoring tumor burden in melanoma patients undergoing treatment with immune checkpoint blockade. J Immunother Cancer 2014;2:42-8. [Crossref] [PubMed]
- Lee JY, Qing X, Xiumin W, et al. Longitudinal monitoring of EGFR mutations in plasma predicts outcomes of NSCLC patients treated with EGFR TKIs: Korean Lung Cancer Consortium (KLCC-12-02). Oncotarget 2016;7:6984-93. [Crossref] [PubMed]
- Zheng D, Ye X, Zhang MZ, et al. Plasma EGFR T790M ctDNA status is associated with clinical outcome in advanced NSCLC patients with acquired EGFR-TKI resistance. Sci Rep 2016;6:20913. [Crossref] [PubMed]
- Chan KC, Yeung SW, Lui WB, et al. Effects of preanalytical factors on the molecular size of cell-free DNA in blood. Clin Chem 2005;51:781-4. [Crossref] [PubMed]
- Swinkels DW, Wiegerinck E, Steegers EA, et al. Effects of blood-processing protocols on cell-free DNA quantification in plasma. Clinical Chemistry 2003;49:525-6. [Crossref] [PubMed]
- El Messaoudi S, Rolet F, Mouliere F, et al. Circulating cell free DNA: Preanalytical considerations. Clin Chim Acta 2013;424:222-30. [Crossref] [PubMed]
- Jung M, Klotzek S, Lewandowski M, et al. Changes in concentration of DNA in serum and plasma during storage of blood samples. Clin Chem 2003;49:1028-9. [Crossref] [PubMed]
- Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet 2016;17:333-51. [Crossref] [PubMed]
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017;14:531-48. [Crossref] [PubMed]


