RAD6: a new target to overcome platinum resistance in ovarian cancer?
Introduction
Ovarian cancer (OC) is a deadly disease, representing the fifth leading cause of cancer death worldwide. In such a context, the major barrier to improve OC survival is resistance to chemotherapy, including platinum. Indeed, the majority of OC patients respond to initial therapy with tumor cytoreductive surgery and platinum-based chemotherapy, but approximately 70% of advanced stage patients will develop tumor recurrence and eventually succumb to recurrent disease, typically characterized by multiple drug resistance (1).
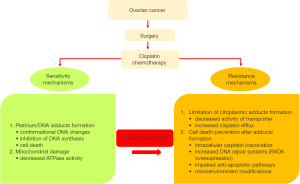
Several mechanisms of cellular resistance to platinum have been proposed in OC and these mechanisms can be classified in two groups: (I) those that block the formation of cytotoxic platinum-DNA adducts; and (II) those that prevent cell death occurring after platinum-DNA adduct formation (Figure 1). Within the second group, impaired activations of anti-apoptotic pathways, enhanced DNA repair mechanisms and microenvironment modifications leading to inhibition of the immune system, metabolic rewiring and acquisition of a stem-like phenotype and epithelial to mesenchymal transition (EMT) contribute to the poor outcome of this disease (2-7). Noteworthy, research on OC stem cells (OCSC) provided novel information on their role in the development of drug resistance and their interplay with environmental conditions (8,9). Indeed, various molecular mechanisms that inhibit apoptosis have been proposed as relevant for cancer stem cell biology and, among others, increased DNA repair mechanisms and remodeling of signaling transduction pathways and cancer cell metabolism. Whether these changes occur primarily in cancer stem cells (CSCs) and are transferred to the progenitor cell populations, or occur de novo in more differentiated cancer cells as well, is still unknown.
RAD6, stemness and drug resistance in OC
The study “RAD6 promotes DNA repair and stem cell signaling in OC and is a promising therapeutic target to prevent and treat acquired chemoresistance” by Somasagara et al., published in Oncogene (10), describes important new findings about novel strategies to overcome chemoresistance, prevent disease recurrence and enhance efficacy of standard chemotherapy.
RAD6 is a ubiquitin-conjugating enzyme upregulated in several human malignancies (e.g., breast, melanoma, and ovarian carcinoma) (11-14) and responsible for the covalent attachment of ubiquitin to other proteins (15) and DNA repair pathways (16). Indeed, much evidence suggests that RAD6 promotes DNA damage tolerance and DNA repair mechanism in response to lethal stimuli, such as chemotherapy (17) and its function has been correlated to progressive disease (11,12), development of a stem-like phenotype and resistance to platinum, a chemotherapeutic agent frequently utilized in OC treatment (14).
In this paper, Somasagara et al. (10) observed the upregulation of RAD6 following carboplatin treatment in parallel with the increased expression and monoubiquitination of DNA damage response (DDR) proteins, such as FANCD2, PCNA, RAD18 and γH2AX. Moreover, RAD6 overexpression correlated with increased levels of CSC markers, e.g., βCatenin, SOX2 and ALDH1A1. Consistently, RAD6 downregulation by specific silencing and its pharmacological inhibition by TZ9 molecule confirmed the relationship between RAD6 expression, chemoresistance and stemness. Noteworthy, RAD6 depletion/inhibition induced: (I) the attenuation of DDR proteins monoubiquitination, responsible for replication stress, thus decreasing DNA repair system efficiency and inhibiting damage tolerance pathways upon carboplatin treatment; (II) the reduction of invasiveness and replication fork velocities; and (III) the downregulation of CSC genes with a parallel inhibition of anchorage-independent growth.
Extremely interesting is the molecular mechanism responsible for RAD6-dependent promotion of CSC gene expression: data suggest that RAD6 works together with RNF20/40 to regulate gene expression by inducing the ubiquitination of H2B (18,19). In turn, the formation of H2B-Ub promotes histone methylation (H3K4 and H3K79) which changes chromatin in an open conformation and causes increased gene transcription in this region (20). Consistently with this interpretation, chromatin immunoprecipitation assays confirmed that RAD6 depletion reduces H2B-Ub levels in ALDH1A1 and SOX2 promoter regions and inhibits the putative βCatenin/TCF binding site of ALDH1A1 and SOX2. Finally, RAD6 directly regulates Wnt/βCatenin signaling, promoting βCatenin stability and its nuclear localization (12).
RAD6 as a molecular target in human ovarian and breast carcinomas and melanomas
The translational relevance of RAD6 inhibition as a strategy to overcome drug resistance has been further evaluated in the context of platinum resistance in OC (10). The authors show that RAD6 inhibition by TZ9 enhances the sensitivity of OC cells to carboplatin, suggesting that combination therapy may be an effective strategy to overtake chemoresistance and prevent disease recurrence in OC. Furthermore, OC cell lines showed enhanced sensitivity to RAD6 inhibition than untransformed ovarian cells, this suggesting that TZ9/carboplatin combination therapy could allow the selective targeting of OC cells and the reduction of chemotherapeutic agent doses, thus minimizing chemotherapy side effects in OC patients. Taken together, these data support the concept that RAD6 inhibition deserves to be evaluated in clinic as a strategy to sustain standard chemotherapy and that RAD6 targeting would be likely more effective than direct Wnt signaling inhibition, since multiple cancer-associated signaling pathways would be affected.
In this scenario, this and other studies have shown that RAD6 is involved in tumor progression in human malignancies (11,12,14,21). Somasagara et al. proposed a prognostic/predictive role of RAD6 as biomarker in OC upon evaluation of a cohort of 26 OC patients, in which RAD6, ALDH1A1 and SOX2 expression were found to be increased after carboplatin/taxane chemotherapy and correlated with poor progression-free survival. This observation provides the rationale to further study this “RAD6 signature” as a biomarker to select OCs poorly sensitive to platinum chemotherapy and, thus, suitable for more aggressive therapeutic strategies. Consistently, RAD6 protein levels were found to be up-regulated in breast hyperplasia, carcinomas and metastases (11) and in primary and metastatic melanomas, but not nevi (13). In breast cancer, RAD6 plays a significant role in maintenance of genomic integrity and an imbalance in the levels and activity of RAD6 leads to chromosomal instability and transformation in vitro. Moreover, a positive and reciprocal regulation between RAD6 and βCatenin was observed: (I) RAD6 is a transcriptional target of βCatenin/TCF complex in normal breast cancer (22); (II) RAD6 is a physiologic regulator of βCatenin ubiquitination and stability (12); (III) increased RAD6 expression contributes to βCatenin stabilization in mammalian cancer cells (12). In addition, RAD6 overexpression in breast cancer subpopulations favors the growth of tumors with homogeneous EMT phenotype, in vivo (23). Finally, a striking upregulation of RAD6 was observed in human melanoma, being its expression negative in most benign melanocytic tumors and positive in 100% of primary and metastatic melanomas (13,21). No positive relationship between RAD6 and βCatenin was observed in benign or malignant melanocytic tumors (13).
CSC metabolism as a therapeutic target in OCSC
Intriguing is the observation that RAD6 targeting in vivo results in reduced DNA repair and damage tolerance mechanisms, inhibition of EMT and stem cell traits and increased carboplatin sensitivity, this suggesting that RAD6 pharmacological inhibition may provide a strategy to overcome drug resistance by targeting CSCs. This hypothesis needs to be discussed in the context of the current literature about OCSCs, which suggests a close relationship between cancer cell metabolism, EMT and platinum resistance (7,8). Recently, it has been reported that CSCs from epithelial OCs, differently from CSCs form other human malignancies (8), privilege oxidative phosphorylation and resist glucose deprivation (7). This observation is consistent with the general view that tumor cells adapt their fate/metabolism to extracellular conditions and modulate the expression of molecular chaperones to this purpose. In such a context, we recently described a role for the HSP90 mitochondrial chaperone TRAP1 in metabolic rewiring and platinum resistance in OC (5). TRAP1 is an antiapoptotic protein involved in drug resistance in several human malignancies (24) and recently described as responsible for suppressing oxidative phosphorylation and enhancing Warburg metabolism in cancer cells (24,25). Indeed, OCs with high TRAP1 expression are characterized by favorable chemotherapy response and lower tumor grade and stage and better overall survival (5,6). Conversely, TRAP1 downregulation in high grade and high stage OCs is associated with higher expression and activity of p70S6K, enhanced cell motility and EMT, increased oxidative metabolism and resistance to platinum (5,6). Notably, this condition of platinum resistance is associated with enhanced production of inflammatory mediators, such as interleukin IL6 and IL8 in OC cells and in patients (5). Altogether, these observations suggest that multiple factors/pathways are involved in the regulation of OCSC plasticity and many of them may be clinically meaningful molecular targets.
In conclusion, while the complexity of molecular mechanisms driving tumor progression and drug resistance in OCs is still a major issue, these recent data support the hypothesis that DNA repair, metabolic rewiring and EMT in CSCs play critical roles in resistance to platinum chemotherapy and tumor relapse after first-line chemotherapy. Thus, this study by Somasagara et al. (10), together with other recent evidences, is starting to decipher the intricate network of proteins that govern the biology of OC cells and OCSCs and to identify new molecular targets for overcoming resistance to platinum in clinics.
Acknowledgments
Funding: This work was supported by AIRC Grant IG2015 Id.16738 to M Landriscina.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Zheng Li (Department of Gynecologic Oncology, The Third Affiliated Hospital of Kunming Medical University, Kunming, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.11.20). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Matsuo K, Lin YG, Roman LD, et al. Overcoming Platinum Resistance in Ovarian Carcinoma. Expert Opin Investig Drugs 2010;19:1339-54. [Crossref] [PubMed]
- Tapia G, Diaz-Padilla I. Molecular Mechanisms of Platinum Resistance in Ovarian Cancer. In: Iván Díaz-Padilla I. editor. Ovarian Cancer - A Clinical and Translational Update. InTech, 2013.
- Thibault B, Castells M, Delord JP, et al. Ovarian cancer microenvironment: implications for cancer dissemination and chemoresistance acquisition. Cancer Metastasis Rev 2014;33:17-39. [Crossref] [PubMed]
- Preston CC, Goode EL, Hartmann LC, et al. Immunity and immune suppression in human ovarian cancer. Immunotherapy 2011;3:539-56. [Crossref] [PubMed]
- Matassa DS, Amoroso MR, Lu H, et al. Oxidative metabolism drives inflammation-induced platinum resistance in human ovarian cancer. Cell Death Differ 2016;23:1542-54. [Crossref] [PubMed]
- Amoroso MR, Matassa DS, Agliarulo I, et al. TRAP1 downregulation in human ovarian cancer enhances invasion and epithelial–mesenchymal transition. Cell Death Dis 2016;7:e2522 [Crossref] [PubMed]
- Pastò A, Bellio C, Pilotto G, et al. Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget 2014;5:4305-19. [Crossref] [PubMed]
- Lupia M, Cavallaro U. Ovarian cancer stem cells: still an elusive entity? Mol Cancer. 2017;16:64. [Crossref] [PubMed]
- Kwon MJ, Shin YK. Regulation of ovarian cancer stem cells or tumor-initiating cells. Int J Mol Sci 2013;14:6624-48. [Crossref] [PubMed]
- Somasagara RR, Spencer SM, Tripathi K, et al. RAD6 promotes DNA repair and stem cell signaling in ovarian cancer and is a promising therapeutic target to prevent and treat acquired chemoresistance. Oncogene 2017;36:6680-90. [Crossref] [PubMed]
- Shekhar MPV, Lyakhovich A, Visscher DW, et al. Rad6 overexpression induces multinucleation, centrosome amplification, abnormal mitosis, aneuploidy, and transformation. Cancer Res 2002;62:2115-24. [PubMed]
- Shekhar MP, Gerard B, Pauley RJ, et al. Rad6B is a positive regulator of beta-catenin stabilization. Cancer Res 2008;68:1741-50. [Crossref] [PubMed]
- Rosner K, Mehregan DR, Kirou E, et al. Melanoma development and progression are associated with Rad6 upregulation and β-catenin relocation to the cell membrane. J Skin Cancer 2014;2014:439205 [Crossref] [PubMed]
- Somasagara RR, Tripathi K, Spencer SM, et al. Rad6 upregulation promotes stem cell-like characteristics and platinum resistance in ovarian cancer. Biochem Biophys Res Commun 2016;469:449-55. [Crossref] [PubMed]
- Lawrence C. The RAD6 DNA repair pathway in Saccharomyces cerevisiae: what does it do, and how does it do it? BioEssays 1994;16:253-8. [Crossref] [PubMed]
- Hoege C, Pfander B, Moldovan GL, et al. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 2002;419:135-41. [Crossref] [PubMed]
- Bailly V, Lamb J, Sung P, et al. Specific complex formation between yeast RAD6 and RAD18 proteins: a potential mechanism for targeting RAD6 ubiquitin-conjugating activity to DNA damage sites. Genes Dev 1994;8:811-20. [Crossref] [PubMed]
- Kao CF, Hillyer C, Tsukuda T, et al. Rad6 plays a role in transcriptional activation through ubiquitylation of histone H2B. Genes Dev 2004;18:184-95. [Crossref] [PubMed]
- Ng HH, Xu RM, Zhang Y, et al. Ubiquitination of histone H2B by Rad6 is required for efficient Dot1-mediated methylation of histone H3 lysine 79. J Biol Chem 2002;277:34655-7. [Crossref] [PubMed]
- Soares LM, Buratowski S. Histone Crosstalk: H2Bub and H3K4 Methylation. Mol Cell 2013;49:1019-20. [Crossref] [PubMed]
- Rosner K, Adsule S, Haynes B, et al. Rad6 is a Potential Early Marker of Melanoma Development. Transl Oncol 2014;7:384-92. [Crossref] [PubMed]
- Shekhar MP, Tait L, Gerard B. Essential role of T-cell factor/beta-catenin in regulation of Rad6B: a potential mechanism for Rad6B overexpression in breast cancer cells. Mol Cancer Res 2006;4:729-45. [Crossref] [PubMed]
- Gerard B, Tait L, Nangia-Makker P, et al. Rad6B acts downstream of Wnt signaling to stabilize β-catenin: Implications for a novel Wnt/β-catenin target. J Mol Signal 2011;6:6. [Crossref] [PubMed]
- Lettini G, Maddalena F, Sisinni L, et al. TRAP1: a viable therapeutic target for future cancer treatments? Expert Opin Ther Targets 2017;21:805-15. [Crossref] [PubMed]
- Amoroso MR, Matassa DS, Agliarulo I, et al. Stress-Adaptive Response in Ovarian Cancer Drug Resistance: Role of TRAP1 in Oxidative Metabolism-Driven Inflammation. Adv Protein Chem Struct Biol 2017;108:163-98. [Crossref] [PubMed]