
Abdominal CT protocol’s influence on postoperative follow-up of lesions detection associated with gastrointestinal tumours
Introduction
Since the advent of computed tomography (CT) in 1973, X-ray CT has taken on more and more important role not just in diagnosing and screening diseases, but also in preoperative planning and postoperative follow-up. This has inadvertently resulted in increased exposure to radiation dose (1). Presently, there are many techniques to reduce the radiation dose for CT scan, including upgrading CT hardware, using automated exposure control, decreasing the number of scan phases, increasing section thickness, developing iterative image reconstruction and post-processing algorithm as well as optimizing scan protocols (2-10). Most vendors offered different models from low-end 16-slice CT to top-tier CT scanners such as more than 256-slice or dual-source CT with potentially lower radiation exposure. Automated tube current modulation could achieve a specified image quality based on subject attenuation or body size, allowing reduction in radiation exposure (9). While CT hardware advances, the development of software such as reconstruction furthers lower radiation exposure. Filtered back projection is a kind of reconstruction method to generate CT images but easily affected by high image noise and artifacts. Statistical iterative image reconstruction method is a new algorithm based on both backward and forward projections according to a statistical metric, showing potentials to significantly improve the reconstructed image quality with lowering voltage or milliampere (7,8). Reducing the scan frequency is the most direct method to reduce radiation exposure without compromising subsequent clinical diagnosis.
Gastrointestinal tumours are very common in the clinic (11). Imaging exam including CT could be used not only in locating the lesions and detecting metastasis, but also for monitoring the therapeutic effects and postoperative follow-up. As gastrointestinal lesions are usually concerned with the wall, CT can offer more evidence for preoperative plan and postoperative follow-up in comparison to other methods (12). In general, abdominal CT imaging is performed with intravenous administration of a kind of nonionic iodine contrast agent including non-enhanced CT (NECT), single-phase enhanced CT (SPCT) and dual-phase enhanced CT (DPCT) (13). The effective dose (ED) associated with DPCT could be more than 20 mSv (5). In this study, we aimed to investigate the diagnostic utility of NECT, arterial phase contrast-enhanced CT (APCT), venous phase contrast-enhanced CT (VPCT), NECT + APCT, NECT + VPCT in the detection and follow-up of relevant lesions in patients with gastrointestinal tumors postoperatively in comparison with NECT + DPCT.
Methods
Patients
A search of our single-hospital pathology database was performed to identify patients who were diagnosed as gastrointestinal tumors between June 2016 and June 2017. Patients were included if they had been performed abdominal CT and undergone surgery as an initial treatment but without history of neoadjuvant therapy and other tumors before surgery. The final study group comprised 200 patients. Histologic type was classified according to the WHO classification for gastric and colorectal cancer and stromal tumors, including 80 cases of stomach cancer (57 cases of adenocarcinoma, 14 cases of signet-ring cell carcinoma, and 9 cases of mucinous adenocarcinoma), 80 cases of colorectal cancer (68 cases of adenocarcinoma, 8 cases of mucinous adenocarcinoma, and 4 cases of signet-ring cell carcinoma), and 40 cases of gastrointestinal stromal tumor (GIST, 25 cases of gastric stromal tumor—19 cases with low-grade malignancy, 5 cases with intermediate malignancy and 1 case with high-grade malignancy; 7 cases of duodenal stromal tumor—5 cases with low-grade malignancy and 2 cases with intermediate malignancy; 5 cases of rectal stromal tumor—4 cases with low-grade malignancy and 1 case with intermediate malignancy; 2 cases of jejunal stromal tumor with intermediate malignancy and 1 case of ileal stromal tumor with high-grade malignancy). Of these 200 patients, 132 were male and 68 were female, with age ranging between 17 and 82 years. The clinical indications of all examinations were determined by a review of the electronic medical record.
This compliant study was approved by the Institutional ethics Review Board of the First Affiliated Hospital, Zhejiang University, and the requirement for informed consent was waived because of the study’s retrospective nature.
Data acquisition
All CT scans were performed on a Philips iCT system (256-slice helical scan; tube voltage = 120 kV, slice thickness = 5 mm, collimation = 0.625 mm ×128 slices, table increment = 97.5 mm/s, pitch = 0.91, the third level iDose image reconstruction). For the contrast agent, 80–100 mL of iohexol was intravenous bolus injection at 3 mL/s through a high pressure injector. The scans were finished on a supine position with both arms up and head first entering the gantry with a protocol including a localizer, NECT scan, arterial phase (30 s after contrast injection) and venous phase (75 s after contrast injection). The scan area is from the diaphragm to the pubic symphysis.
ED calculation
Volume CT dose index (CTDIvol) and dose length product (DLP) could be retrieved from the dose report of each scan. ED of each scan could be calculated by multiplying DLP by a dose conversion factor. According to the European Guidelines on Quality Criteria for Computed Tomography, the dose conversion factor of 0.015 for the abdomen was used in this study (14).
Image reading
CT images of each patient were separated into six groups: NECT, APCT, VPCT, NECT + APCT, NECT + VPCT and NECT + DPCT. These images were independently analyzed by two radiologists who had more than 5-year experience in reading abdominal CT films. The anastomosis (e.g., presence of anastomotic stenosis, thickening of walls at the point of anastomosis and recurrence of tumors), lymphadenopathy and liver metastasis were evaluated. Any discrepancy of film reading was resolved by the two radiologists in consensus.
Statistical analysis
Cohen’s Kappa statistics and Pearson chi-square statistics were performed to compare values of each study group with NECT + DPCT group by SPSS software version 16.0. The ED differences among these six groups were analyzed by one-way ANOVA. P<0.05 was considered as statistically significant.
Results
Lesions concerned with gastrointestinal tumors were anastomosis, metastases and lymphadenopathy
Anastomosis
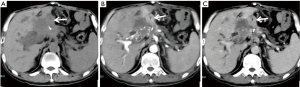
Fifteen cases of wall thickening were displayed at NECT + DPCT, including 5 cases of gastric anastomosis, 5 cases of Roux-en-Y anastomosis, 3 cases of rectal anastomosis and 2 cases of ascending colon anastomosis. Three out of them were confirmed tumor relapse. No abnormality was found in 185 cases in terms of anastomosis. The results of anastomosis detected in each group were listed in Table 1. There were no significant differences among these six groups (P>0.05). A typical case of gastric anastomosis found in NECT, APCT and VPCT was shown in Figure 1.

Full table
Metastatic lesions
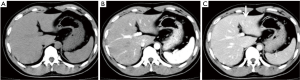
Forty cases of metastatic lesions were displayed at NECT + DPCT. However, NECT could only show 15 cases of metastatic lesions, while APCT and NECT + APCT could show 32 of such cases with false negative rates of 62.5% and 20%, respectively. Out of the 40 metastatic lesions, 30 cases were liver metastases, 4 cases were abdominal metastases, 4 cases were omentum and peritoneal metastases and 2 cases were adrenal metastases (Table 2). Liver metastases were mistaken as low density lesions at NECT. VPCT detected an equal number of metastatic lesions in comparison with NECT + DPCT. A case of low density lesion was shown in Figure 2. The lesion could be displayed at VPCT (Figure 2C), but not shown at both NECT (Figure 2A) and APCT (Figure 2B).

Full table
Lymphadenopathy
Five cases of lymphadenopathy were not displayed at NECT, resulting in false negative rate at 68.8%. Eight cases of lymphadenopathy could not be detected by APCT and NECT + APCT, the false negative rate of which was 43.8%. VPCT was comparable to NECT + DPCT in detecting lymphadenopathy (Table 3).

Full table
ED
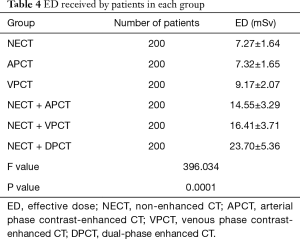
The EDs received by all patients in each group were listed in Table 4. Based on the LSD t-test, the difference between the NECT and APCT was not statistically significant (P=0.91). However, the differences between each other for the rest of the groups were statistically significant (P<0.001). Compared with NECT + DPCT (23.70±5.36), ED of VPCT protocol decreased at 61% (9.17±2.07).
Full table
Discussion
With the wide application of CT in the diagnosis of disease, radiation associated with CT is becoming a big public concern (15,16). It has noted that although CT only accounts for 7% of all radiological examinations between 1950 and 2007, the cumulative ED from CT exceeds all other radiological examinations by 40% (17). It should also be noted that carrying out full abdominal CT reviews after performing surgery for gastrointestinal tumors is necessary, but such reviews expose patients to higher than required radiation dose due to the multi-phase nature of abdominal contrast-enhanced CT. In addition, a certain tube current and voltage is required to mitigate the presence of intestinal gas (18,19). Development of statistical reconstruction algorithms is one of the most active researches in lowering radiation exposure in CT examination (5,6). Although iterative CT reconstruction based on statistical theory could reduce image noise with lowering radiation, it is challenging to get the optimal iterative number. With the increase of iteration number, image noise reduce at a cost of lowering contrast and worse structure delineation. We aimed to investigate the utility of different phases of CT for monitoring patients with gastrointestinal tumor postoperatively. Images of NECT and DPCT in each patient were divided into six groups including NECT, APCT, VPCT, NECT + APCT, NECT + VPCT and NECT + DPCT. The diagnostic accuracy of each group was compared to the standard protocol of NECT + DPCT. Based on the results, we observed a reduction of ED in patients undergoing VPCT for their postoperative follow-up and evaluation by 61.3% in comparison with NECT + DPCT without any influences on patients’ management.
NECT has a reduced contrast-noise ratio and reduced spatial resolution. This may reduce the ability to detect and characterize liver lesions. CECT scan of the liver allows evaluation of lesion enhancement for specific characterization of liver masses and improves lesion detection as compared with NECT. In addition, the lesions’ relationship to vascular structures can be assessed after administration of intravenous iodinated contrast. The main purpose of conventional NECT + DPCT protocol for postoperative follow-up of gastrointestinal tumors is to detect anastomotic recurrence, swollen lymph nodes, and metastatic lesions as well. Our study has observed that in comparison with NECT + DPCT, there were no differences to detect anastomotic recurrences for NECT, APCT, VPCT, NECT + APCT and NECT + VPCT. As for detection of swollen lymph nodes, NECT had a false negative rate of 68.8%, APCT and NECT + APCT both recorded a false negative rate of 43.8%, while VPCT showed no significant differences in false negative rates when compared to NECT + VPCT and NECT + DPCT; for metastatic lesions, NECT had a false negative rate of 62.5%, APCT and NECT + APCT both stood at 20%, while VPCT showed no significant differences in false negative rates when compared to NECT + VPCT and NECT + DPCT. However, the effective absorbed dose of VPCT was measured to be 9.17±2.07 mSv, while that for NECT + DPCT was approximately 2.6 times greater at 23.70±5.36 mSv, and for NECT + APCT was approximately 1.6 times greater at 14.55±3.29 mSv, and for NECT + VPCT was approximately 1.8 times greater at 16.41±3.71 mSv. There were no significant differences in the effective absorbed dose among NECT, APCT and VPCT.
In addition, with respect to detecting anastomotic recurrences, there were no significant differences in image quality between VPCT and NECT + DPCT. Only VPCT may be sufficient for postoperative follow-up and evaluation for gastrointestinal tumors, which greatly reduce the radiation exposure to patients.
There are some limitations in this study. The patients were only recruited from one hospital and CT scanner was only from a company. In addition, influences on the ED with different collimations should be investigated further.
In conclusion, VPCT may be sufficient to detect relevant lesions for postoperative follow-up of gastrointestinal tumors, with much less radiation exposure in comparison with conventional NECT + DPCT.
Acknowledgments
Funding: This study was supported by a grant from Project co-sponsored by Zhejiang Province and Health & Family Planning Commission of China (WSK 2014-2-009).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yì-Xiáng J. Wáng, Yong Wang) for the series “Translational Imaging in Cancer Patient Care” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.12.05). The series “Translational Imaging in Cancer Patient Care” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This compliant study was approved by the Institutional Ethics Review Board of the First Affiliated Hospital, Zhejiang University, and the requirement for informed consent was waived because of the study’s retrospective nature.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med 2007;357:2277-84. [Crossref] [PubMed]
- Kubo T, Ohno Y, Kauczor HU, et al. Radiation dose reduction in chest CT--review of available options. Eur J Radiol 2014;83:1953-61. [Crossref] [PubMed]
- Costello JE, Cecava ND, Tucker JE, et al. CT radiation dose: current controversies and dose reduction strategies. AJR Am J Roentgenol 2013;201:1283-90. [Crossref] [PubMed]
- Chaikh A, Balosso J. Quantitative comparison of dose distribution in radiotherapy plans using 2D gamma maps and X-ray computed tomography. Quant Imaging Med Surg 2016;6:243-9. [Crossref] [PubMed]
- Tamm EP, Rong XJ, Cody DD, et al. Quality initiatives: CT radiation dose reduction: how to implement change without sacrificing diagnostic quality. Radiographics 2011;31:1823-32. [Crossref] [PubMed]
- Gill MK, Vijayananthan A, Kumar G, et al. Use of 100 kV versus 120 kV in computed tomography pulmonary angiography in the detection of pulmonary embolism: effect on radiation dose and image quality. Quant Imaging Med Surg 2015;5:524-33. [PubMed]
- Willemink MJ, de Jong PA, Leiner T, et al. Iterative reconstruction techniques for computed tomography Part 1: technical principles. Eur Radiol 2013;23:1623-31. [Crossref] [PubMed]
- Willemink MJ, Leiner T, de Jong PA, et al. Iterative reconstruction techniques for computed tomography part 2: initial results in dose reduction and image quality. Eur Radiol 2013;23:1632-42. [Crossref] [PubMed]
- Choi KS, Kim SH, Kim SG, et al. Early Gastric Cancers: Is CT Surveillance Necessary after Curative Endoscopic Submucosal Resection for Cancers That Meet the Expanded Criteria? Radiology 2016;281:444-53. [Crossref] [PubMed]
- Chaikh A, Balosso J. Correlation between pneumonitis risk in radiation oncology and lung density measured with X-ray computed tomography. Quant Imaging Med Surg 2016;6:413-7. [Crossref] [PubMed]
- Gong J, Kang W, Zhu J, et al. CT and MR imaging of gastrointestinal stromal tumor of stomach: a pictorial review. Quant Imaging Med Surg 2012;2:274-9. [PubMed]
- Kang B, Lee JM, Song YS, et al. Added Value of Integrated Whole-Body PET/MRI for Evaluation of Colorectal Cancer: Comparison With Contrast-Enhanced MDCT. AJR Am J Roentgenol 2016;206:W10-20 [Crossref] [PubMed]
- Kalkmann J, Zeile M, Antoch G, et al. Consensus report on the radiological management of patients with gastrointestinal stromal tumours (GIST): recommendations of the German GIST Imaging Working Group. Cancer Imaging 2012;12:126-35. [Crossref] [PubMed]
- Jang J, Jung SE, Jeong WK, et al. Radiation Doses of Various CT Protocols: a Multicenter Longitudinal Observation Study. J Korean Med Sci 2016;31:S24-31. [Crossref] [PubMed]
- D'Ambrosio L, Palesandro E, Boccone P, et al. Impact of a risk-based follow-up in patients affected by gastrointestinal stromal tumour. Eur J Cancer 2017;78:122-32. [Crossref] [PubMed]
- Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology 2009;251:175-84. [Crossref] [PubMed]
- Fuentes-Orrego JM, Sahani DV. Low-dose CT in clinical diagnostics. Expert Opin Med Diagn 2013;7:501-10. [Crossref] [PubMed]
- Plumb AA, Kochhar R, Leahy M, et al. Patterns of recurrence of gastrointestinal stromal tumour (GIST) following complete resection: implications for follow-up. Clin Radiol 2013;68:770-5. [Crossref] [PubMed]
- Joensuu H, Reichardt P, Eriksson M, et al. Gastrointestinal stromal tumor: a method for optimizing the timing of CT scans in the follow-up of cancer patients. Radiology 2014;271:96-103. [Crossref] [PubMed]


