
Clinicopathological and theranostic analysis of 82 breast cancer patients older than 80 years
Introduction
Breast cancer is the most common malignant tumor in women. The incidence of breast cancer has been rising worldwide (1). Age is one of the major risk factors for breast cancer: more than 30% of all new breast cancers occur in women aged 70 years or more. Furthermore, breast cancer–related mortality increases with age (2,3). And according to the data of the Slovenian Cancer Registry for 2008, 11% of all breast cancer patients were aged more than 80 years (4). In contrast, only 15% of the cases in women older than 65 years in Chinese population (5). However, with an expanding aging population, advancing industrial and medical infrastructures, increasing awareness of cancer prevention, and on-going breast cancer screening in our population, there is an increase in breast cancer rate among older women in China (6). Elderly patients are unique due to their physical state, natural life span and poor endurance and prognosis for surgical operations and chemotherapy. However, current clinical research related to population >80 years old is very limited (7), and there is no consensus or guidelines on how to treat elderly breast cancer patients (8). Thus, it is very important to pay close attention to the clinical manifestations of elderly breast cancer patients. As we enter the current era of evidence-based medicine, research suggests cancer patients >80 years old can benefit from standard treatments (9,10). In this study, we performed retrospective analysis and follow-up of clinical data from 82 breast cancer patients with age 80 and older, and discuss the clinical features and treatment strategies.
Methods
Clinical and pathology data from 82 cases of breast cancer patients >80 years old women that were diagnosed in our hospital from June 2009 to June 2017 and confirmed with pathology were collected and analyzed. Clinical features were retrospectively analyzed and accompanied by follow-ups with phone calls. Up until 6/30/2017, median follow-up time was 50 months.
Results
General
Among total number of breast cancer patients of this period, 3.8% patients aged >80 years old (82/2, 180). Average age at diagnosis of this elderly group is 82.9 years, with the oldest being 94. Disease duration range from 1 month to 12 years, mean disease duration of 27.9 months. Initial symptoms include 78 cases (95.1%) reported as breast lumps without pain, 1 case of nipple erosion, 1 case nipple discharge, 2 cases lump and discharge. 14 cases (17.1%) of breast lump skin invasion, 5 cases of nipple depression, 2 cases of breast skin dimpling, 5 cases of multiple lumps. Tumor size ranges from 0.5 to 10 cm, average 3.0 cm. Forty-five on the left side and 37 on the right side. Forty-eight cases (58.5%) located in the upper-outer quadrant, 22 cases in the upper-inner quadrant, 12 cases in either lower-inner or lower-outer quadrant. Sixty-one cases (74.4%) had associated diseases, including hypertension (44 cases) and diabetes (16 cases). Thirty-eight cases (46.3%) had a history of surgery with 1 case of contralateral breast cancer surgery. One case reported family history of breast cancer with the mother. Average hospital stay prior to surgery is 5 days (2 to 11 days). Average inpatient stay is 13 days (5 to 28 days), one case for pacemaker implantation prior to surgery, seven cases ICU care post-surgery.
Pathological diagnosis
Pathological analysis was performed in our Pathology department, and classified according to WHO standards (11) (Table 1).
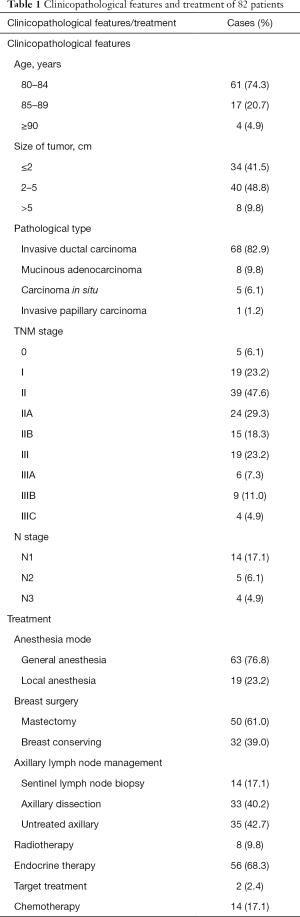
Full table
Clinical stage
Patients were staged according to the American Joint Committee on Cancer (AJCC) classification (12) (Table 1).
Immunohistochemistry and Her2 FISH analysis
Seventy-seven of the 82 samples were tested according to ASCO guidelines. Tests include expression of oestrogen receptor expression (13), progesterone receptor (13), human epidermal growth factor receptor 2 (14) and Ki67 (15). When the immunohistochemistry (IHC) result is 2+, FISH were used to retest, and 2013 updated St Gallen subtypes were applied (16) (Table 2).

Full table
Treatment strategies
All 82 cases applied combination therapy of surgical tumor resection. Specific surgical methods are shown in Table 1. Fourteen cases had chemotherapy post-surgery, seven received docetaxel plus cyclophosphamide (TC) and seven received Xeloda. Eight cases had radiotherapy, 56 cases received hormone therapy post-surgery, 50% of which stopped due to side effects. Two cases of the Her2 positive patients received targeted treatment.
Follow-up
After the surgical procedure, the patients were followed up from 0.1 to 8.0 years. During this period, 18 cases died, 10 of which died of breast cancer, while 8 of which died of other causes. Four of which had cancer metastasis to liver and bone, two cases were luminal B type of breast cancer, two were triple negative. Ten had relapse, seven of which were triple negative, two was Her2 positive and one luminal B type, seven of which received partial mastectomy, three cases received total mastectomy.
Discussion
Clinical and pathological features
Long disease course, late treatment
The longest disease course in this group is 12 years. In average, it took 2 years for the patients come into the clinic, thus the treatment is usually late. Nearly 20% (14/82) of the patients already had skin invasion and even necrosis at the time of diagnosis, 5 cases of nipple depression, about 10% (8/82) had tumor >5 cm, close to 25% (19/82) are locally advanced or advanced tumor, which is consistent with other studies (17,18). Ali et al. (19) reported that in patients ≥75 years old, 11% had tumor >5 cm at the time of diagnosis. This could be due to the clinical manifestation of lump without pain, slow tumor growth, no impact on normal life. Lack of health awareness, concerns for the burden on their children lead to their reluctance and delay to seek treatment. Moreover, limited attention for breast cancer screen in elderly women is another important reason for the delayed diagnosis and treatment of this group (20). None of the patients from this group was identified from mammography or ultrasound, suggesting a breast cancer screen is likely to be useful to improve the identification of disease in early stages and promote the long-term survival of the patients (21). However, screening mammography in patients aged 80 years or more is still controversial (22-24).
Multiple associated diseases
Elderly patients usually have various systemic diseases. The rate of associated disease is 74.4% (61/82), with most common one as diabetes and hypertension. This might be one of the reasons for their reluctance to seek treatments, as they concern about their physical weakness and impact of surgical operation. Schrauder (25) has observed that type 2 diabetes is often associated with more advanced cancer in older women and Jung (26) has found that even hypertension can be an adverse prognostic factor. According to the reports (27,28), the mortality rate for breast cancer surgery is very low, 0–3%. Therefore, the factors affect surgical treatment is not age but the associated diseases. It is necessary for elderly patients to go through comprehensive pre-admission testing prior surgery, blood gas testing, pulmonary function test, 24 hr dynamic electrocardio graph are all necessary to make sure the normal function of heart and lung. It is recommended to seek consultations for risk assessment, effective communications with family members prior to surgery and ICU care post-surgery. This group of 82 patients did not show any severe complications or death related to the surgery. Only one patient was found to be bleeding into the wound and another patient developed deep vein embolism of lower limb. In sum, sufficient preparation before the surgery, close monitor and care during and after the surgery, and a proper management of associated diseases could improve the endurance and safety of the surgical operation for the elderly patients.
Pathological manifestations
In this group of patients, 93.9% (77/82) were invasive, among which 88.3% (68/77) were invasive ductal carcinoma, higher than previous report (29). Relatively higher incidence of mucinous carcinoma, 10.4% (8/77). It is generally reported (30,31) breast cancer in elderly is less invasive, slow growing, low rate in lymph node metastasis, better differentiated, lower Ki-67 rate, higher receptor positive rate, and lower Her2 positive rate. These features are mostly consistent with the pathological features of this group of patients. In this group, there are 29.9% (23/77) of lymph node metastasis, vessel tumor emboli 26.8% (22/82) and 56.1% (46/82) with Ki-67 expression lower than 30%.
This group of 82 cases invasive carcinoma has a high rate of triple negative cases, close to 1/3 (25/82), higher than previously reported (32) 10–23.8% in our country. This might be due to neoadjuvant endocrine therapy after being diagnosed as hormone positive cancer by core needle biopsy and refused to do surgery. Meanwhile, during the follow-up visit, in the 10 cases that had relapse or metastasis related to breast cancer, 70% (7/10) were triple negative. This indicates a higher risk of relapse and metastasis for triple negative cancer, poor prognosis in the elderly patients. Chemotherapy and total mastectomy should be considered.
Her2 positive case is 7.3% (6/82) in this group, lower than 20% as previously reported (33), luminal type 56.1% (46/82) lower than reported (34), in which 45.7% (21/46) is luminal A type, 5 cases were ER+PR-, the rest were double positive.
Surgical and anesthesia procedure
There are no specific recommendations in the literature concerning the extent of surgical procedure performed in breast cancer patients aged 80 years or older (35). Many elderly patients give up on surgical treatment due to high risk assessment for this type of operation on this particular group (36). Wildiers et al. (37) showed that surgery + tamoxifen combination is better than Tamoxifen alone in lowering the local relapse and improving survival. Therefore, earlier surgery is recommended for elderly patients who can tolerate. However, the surgical procedure is still not standardized (38,39).
Breast surgery
We found this group of 82 cases were more likely to receive mastectomy in the place of breast conserving surgery, 50 cases received mastectomy, while 32 cases received breast conservative surgery, is similar to other results (40,41). The high rate of mastectomy can be explained by the fact that elderly patients have more advanced stage breast cancer. On the other hand, mastectomy does spare elderly patients from adjuvant radiation (42). We analyzed the 10 relapse cases, in which 7 went through extended lumpectomy, 3 cases resection. These people did not receive radiotherapy due to their age and higher triple negative rate led to poor disease control post breast conservative operation. The relapse rate was 21.9% (7/32) among those people who received lumpectomy, much higher than the finding by Hughes et al. (43). We recommend core needle biopsy prior to surgery, in combination with pathological classification and immunohistochemistry analysis. If it is high grade malignancy, total mastectomy will be considered to avoid the high risk of relapse.
Axillary treatment
Forty-seven cases in this group via axillary dissection or sentinel lymph node biopsy to confirmed lymph node metastasis or not. About 42.7% (35/82) of the patients left untreated due to negative results or poorer physical status. Martelli et al. (44) have studied the possibility to spare lumpectomy to older women in a randomized trial and found just a 2% of clear axillary metastatic involvement at 5-year follow up. However, let’s think about old ladies surviving more than 10 years to a conservative surgery without axillary dissection and then be devastated by a rapidly worsening monstrous lymph edema sustained by axillary recurrence, without a chance of relief. As mentioned above, about 28% (23/82) had lymph node metastasis. The percentage could be higher in reality. According to our experiences, we recommend axillary dissection for those who had confirmed axillary lymph node metastasis, others receive sentinel lymph node biopsy depend on their tolerance state, this could facilitate prognosis assessment, decide complementary therapy options, and avoid the situation of not knowing what to do when metastatic lymph node occurs down the road. With more experiences with the surgical operation, sentinel lymph node biopsy takes about 10 min, 2 cm incision, which will not increase surgical injury.
Anesthesia
Eight cases in this group underwent a total mastectomy for cancer under local anesthesia. All of them had low tolerance levels for general anesthesia, smaller lump, negative in axillary examination. They all had a good surgical outcome. In the 63 cases underwent general anesthesia, six had delirium and recovered from Olanzapine orally without any other complications from the anesthesia.
In short, we think it is better to perform radical excision under intensive care, if the elderly patient does not have severe associated diseases. The operation procedure should include total mastectomy and sentinel lymph node biopsy, meanwhile limit the length of surgical procedure. For those who have multiple associated diseases, poor endurance for surgery, and early disease onset, the procedure area can be contracted.
Systemic therapy
Because of multiple associated diseases, poor physical state and organ degeneration in the elderly, multiple factors need to be taken into consideration when deciding on a reasonable treatment option.
Endocrine therapy
There are 68.3% (58/82) cases received endocrine therapy, mainly aromatase inhibitors. However, follow-up data show 50% of the patients voluntarily withdraw medication due to side effect such as arthralgia, lack of attention and affordability. Endocrine therapy usually has higher efficacy and lower side effects. Elderly breast cancer patients have higher positive rate of hormone receptors, so it is especially important to use complementary endocrine therapy (45). We think, because elderly patients older than 80 often accompany cardiovascular diseases or osteoporosis, tamoxifen alone or tamoxifen followed by aromatase inhibitors could be beneficial and detoxifying to the patients. It is advised to emphasize the importance of endocrine therapy and scheduled follow-ups to patients’ family members and the patients themselves.
Chemotherapy
In this group, 17% (14/82) of the patients received chemotherapy. Chemotherapy drugs are mainly capecitabine alone or docetaxel in combination with cyclophosphamide. The seven capecitabine cases were well tolerated for 4–6 treatments, two of which showed hand-foot syndrome and recovered after targeted treatment. Five TC cases did not finish the treatment due to side effects such as bone marrow suppression. Currently, there is no large scale randomized clinical trial for chemotherapy in elderly breast cancer patients or standardized practice. Renal function and bone marrow reserve decrease with age and can increase the risk of toxic effects from treatment regimens (46). Therefore, if it is necessary to use complementary chemotherapy, we need to take into consideration that survival benefit and toxicity associated with chemotherapy, in order to select more effective and convenient approaches with less side effects (47). Treatment guidelines do not set an upper age limit for the use of chemotherapy, but acknowledge that comorbid medical conditions and life expectancy must be considered when prescribing chemotherapy (48).
Radiotherapy
Due to a high proportion of patients who underwent mastectomy, irradiation was performed only in 9.8% of our patients. In the young age group, the patients who underwent breast conservative surgery (BCS) were recommended to receive adjuvant radiation therapy to the remaining breast tissue (49). While in the old age group, radiotherapy could be avoided in case of a large surgical margin, a tumor smaller than 2 cm, a low or moderate tumor grade and hormone-dependent (50,51).
Finally, several limitations of this study should be taken into account. Because this is a retrospective observational study with a small number of patients, there is undoubtedly selection bias and residual confounding by factors for which we do not have data. Most physicians take comorbid conditions into account when choosing treatment strategies, while whether these comorbidities influence the biology of breast cancer leading to recurrence or survival still unknown (52,53). Functional imaging may define tumor biological characteristics (54,55), and help therapeutic planning with personalized medicine (56).
Conclusions
Breast cancer in elderly is not definitely a less aggressive disease compared with the cancer arising in younger women. There are unique clinical and pathological features associated with elderly breast cancer patients, such as long disease course, late on-set and multiple associated diseases. Meanwhile, high rate for triple negative, low rate for Her2 positive, poor endurance for chemotherapy and compliance for endocrine therapy, while surgery and endocrine therapy are the most common and effective treatment strategies. It is true that many 80 years or older patients receive less than standard surgical treatment (57). Mastectomy was performed more frequently, and axillary dissection and radiation were performed less frequently, among older patients, consistent with other reports (41).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yì-Xiáng J. Wáng, Yong Wang) for the series “Translational Imaging in Cancer Patient Care” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.11.24). The series “Translational Imaging in Cancer Patient Care” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study received the approval of local ethics committee. Written informed consent was obtained from each patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- van de Water W, Markopoulos C, van de Velde CJ, et al. Association between age at diagnosis and disease-specific mortality among postmenopausal women with hormone receptor-positive breast cancer. JAMA 2012;307:590-7. [Crossref] [PubMed]
- Kimmick GG, Balducci L. Breast cancer and aging: clinical interactions. Hematol Oncol Clin North Am 2000;14:213-34. [Crossref] [PubMed]
- Primic Zakelj M. Cancer in Slovenia 2008. Ljubljana: Institute of Oncology Ljubljana, Epidemiology and Cancer Registy, 2011.
- Yang MT, Rong TH, Huang ZF, et al. Clinical analysis of resectable breast cancer: a report of 6263 cases. Chin J Cancer 2005;24:327-31. [PubMed]
- Wei Z, Bin X, Wei L, et al. Cancer incidence trend among elderly people in urban Shanghai in 1973-1999. Chin J Geriatr 2005;24:701-4.
- Kemeny MM, Peterson BL, Kornblith AB, et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol 2003;21:2268-75. [Crossref] [PubMed]
- National Comprihensive Cancer Network, Breast cancer. Version 3.2012. Available online: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in old women with early-stage breast cancer. N Engl J Med 2009;360:2055-65. [Crossref] [PubMed]
- Barthélémy P, Hertz D, Mathelin C, et al. Adjuvant chemotherapy in elderly patients with early breast cancer. Impact of age and comprehensive geriatric assessment on tumor board proposals. Crit Rev Oncol Hematol 2011;79:196-204. [Crossref] [PubMed]
- IakhaniSR, Ellis IO, Schnitt SJ, et al. WHO classification of tumors of the breast. World Health Organization classification of tumors. 4th ed, Lyon: IARC Press, 2012.
- Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual, 7th ed. New York: Springer, 2010:347-76.
- Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784-95. [Crossref] [PubMed]
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J Clin Oncol 2013;31:3997-4013. [Crossref] [PubMed]
- Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst 2011;103:1656-64. [Crossref] [PubMed]
- Untch M, Gerber B, Harbeck N, et al. 13th st. Gallen international breast cancer conference 2013: primary therapy of early breast cancer evidence, controversies, consensus - opinion of a german team of experts (zurich2013). Breast Care (Basel) 2013;8:221-9. [PubMed]
- Louwman WJ, Vulto JC, Verhoeven RH, et al. Clinical epidemiology of breast cancer in the elderly. Eur J Cancer 2007;43:2242-52. [Crossref] [PubMed]
- Gennari R, Curigliano G, Rotmensz N, et al. Breast carcinoma in elderly women: features of disease presentation, choice of local and systemic treatments compared with younger postmenopausal patients. Cancer 2004;101:1302-10. [Crossref] [PubMed]
- Ali AM, Greenberg D, Wishart GC, et al. Patient and tumour characteristics, management, and age-specific survival in women with breast cancer in the East of England. Br J Cancer 2011;104:564-70. [Crossref] [PubMed]
- Zapka JG, Stoddard AM, Costanza ME, et al. Breast cancer screening by mammography: utilization and associated factors. Am J Public Health 1989;79:1499-502. [Crossref] [PubMed]
- Vetter M, Huang DJ, Bosshard G, et al. Breast cancer in women 80 years of age and older: a comprehensive analysis of an underreported entity. Acta Oncol 2013;52:57-65. [Crossref] [PubMed]
- Schonberg MA, McCarthy EP. Mammography screening among women age 80 years and older. Consider the risks. J Clin Oncol 2009;27:640-1. [Crossref] [PubMed]
- Badgwell BD, Giordano SH, Duan ZZ, et al. Mammography before diagnosis among women age 80 years and older with breast cancer. J Clin Oncol 2008;26:2482-8. [Crossref] [PubMed]
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA 2001;285:2750-6. [Crossref] [PubMed]
- Schrauder MG, Fasching PA, Häberle L, et al. Diabetes and prognosis in a breast cancer cohort. J Cancer Res Clin Oncol 2011;137:975-83. [Crossref] [PubMed]
- Jung SY, Rosenzweig M, Linkov F, et al. Comorbidity as a mediator of survival disparity between younger and older women diagnosed with metastatic breast cancer. Hypertension 2012;59:205-11. [Crossref] [PubMed]
- Samain E, Schauvliège F, Deval B, et al. Anesthesia for breast cancer surgery in the elderly. Crit Rev Oncol Hematol 2003;46:115-20. [Crossref] [PubMed]
- Tao W, Fei J. Basic principles and advances in the diagnosis and treatment of breast cancer in the elderly. Chinese journal of Multiple Organ Diseases in the Elderly 2009;8:120-2.
- Hong Z, He X, Qing L, et al. Clinical analysis on breast cancer patients over 70 years of age. Chin J N Clin Med 2006;28:385-8.
- Pierga JY, Girre V, Laurence V, et al. Characteristics and outcome of 1755 operable breast cancer in women over 70 years of age. Breast 2004;13:369-75. [Crossref] [PubMed]
- Wildiers H, Kunkler I, Biganzoli L, et al. Management of breast cancer in elderly individuals: recommendations of the International Society of Geriatric Oncology. Lancet Oncol 2007;8:1101-15. [Crossref] [PubMed]
- Guan Y, Xu BH. Analysis of clinicopathological characteristics and prognosis for triple negative breast cancer: a report of 108 eases. Zhonghua Zhong Liu Za Zhi 2008;30:196-9. [PubMed]
- Morales L, Reigosa A, Caleiras E, et al. HER2/neu expression in Venezuelan patients with locally advanced breast cancer. Invest Clin 2008;49:69-78. [PubMed]
- Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst 2001;93:65-6. [Crossref] [PubMed]
- Van Leeuwen BL, Rosenkranz KM, Feng LL, et al. The effect of under-treatment of breast cancer in women 80 years of age and older. Crit Rev Oncol Hematol 2011;79:315-20. [Crossref] [PubMed]
- Schonberg MA, Marcantonio ER, Ngo L, et al. Causes of death and relative survival of older women after a breast cancer diagnosis. J Clin Oncol 2011;29:1570-7. [Crossref] [PubMed]
- Wildiers H, Kunkler I, Biganzoli L, et al. Management of breast cancer in elderly individuals: recommendations of the international society of geriatric oncology. Lancet Oncol 2007;8:1101-15. [Crossref] [PubMed]
- Odendaal Jde V, Apffelstaedt JP. Limited surgery and tamoxifen in the treatment of elderly breast cancer patients. World J Surg 2003;27:125-9. [PubMed]
- Arriagada R, Lê MG, Contesso G, et al. Predictive factors for local recurrence in 2006 patients with surgically resected small breast cancer. Ann Oncol 2002;13:1404-13. [Crossref] [PubMed]
- Yamada A, Narui K, Sugae S, et al. Operation with less adjuvant therapy for elderly breast cancer. J Surg Res 2016;204:410-7. [Crossref] [PubMed]
- Schonberg MA, Marcantonio ER, Li D, et al. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol 2010;28:2038-45. [Crossref] [PubMed]
- Wang J, Kollias J, Boult M, et al. Patterns of surgical treatment for women with breast cancer in relation to age. Breast J 2010;16:60-5. [Crossref] [PubMed]
- Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or order with early breast cancer. N Engl J Med 2004;351:971-7. [Crossref] [PubMed]
- Martelli G, Boracchi P, de Palo M, et al. A randomized trial comparing axillary dissection to no axillary dissection in older patients with T1N0 breast cancer: results after 5 years of follow-up. Ann Surg 2005;242:1-6. [Crossref] [PubMed]
- Osborn G, Jones M, Champ C, et al. Is primary endocrine therapy effective in treating the elderly, unfit patient with breast cancer? Ann R Coll Surg Engl 2011;93:286-9. [Crossref] [PubMed]
- Lichtman SM, Skirvin JA. Pharmacology of antineoplastic agents in older cancer patients. Oncology 2000;14:1743-55. [PubMed]
- Crivellari D, Aapro M, Leonard R, et al. Breast cancer in the elderly. J Clin Oncol 2007;25:1882-90. [Crossref] [PubMed]
- McDermott AM, Toelle TR, Rowbotham DJ, et al. The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain 2006;10:127-35. [Crossref] [PubMed]
- Fisher B, Redmond C, Poisson R, et al. Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med 1989;320:822-8. [Crossref] [PubMed]
- Kunkler IH, Williams LJ, Jack WJ, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 2015;16:266-73. [Crossref] [PubMed]
- Chaikh A, Balosso J. Quantitative comparison of dose distribution in radiotherapy plans using 2D gamma maps and X-ray computed tomography. Quant Imaging Med Surg 2016;6:243-9. [Crossref] [PubMed]
- Hancke K, Denkinger MD, Konig J, et al. Standard treatment of female patients with breast cancer decreases substantially for women aged 70 years and older: a German clinical cohortstudy. Ann Oncol 2010;21:748-53. [Crossref] [PubMed]
- Hidrovo I, Dey J, Chesal ME, et al. Experimental method and statistical analysis to fit tumor growth model using SPECT/CT imaging: a preclinical study. Quant Imaging Med Surg 2017;7:299-309. [Crossref] [PubMed]
- Yuan J, Wong OL, Lo GG, et al. Statistical assessment of bi-exponential diffusion weighted imaging signal characteristics induced by intravoxel incoherent motion in malignant breast tumors. Quant Imaging Med Surg 2016;6:418-29. [Crossref] [PubMed]
- Ha R, Mema E, Guo X, et al. Quantitative 3D breast magnetic resonance imaging fibroglandular tissue analysis and correlation with qualitative assessments: a feasibility study. Quant Imaging Med Surg 2016;6:144-50. [Crossref] [PubMed]
- Wang YX, Lin J. Preface to 2017 focused issue: quantitative imaging of thoracic diseases. J Thorac Dis 2017;9:4723. [Crossref] [PubMed]
- Crivellari D, Spazzapan S, Lombardi D, et al. Breast cancer in the elderly: treatment of 1500 patients. Breast J 2006;12:353-9. [Crossref] [PubMed]


