Expression and significance of EMMPRIN, MMP-9 and TIMP-1 in non-small cell lung carcinoma tissues of Guangxi Province, China
Introduction
The invasion and metastasis are the most important biological characteristics of malignant tumors, and also are the major reasons for patient treatment failure and mortality. The invasion and metastasis of lung cancer is a multi-stage, multi-step, sequential process involved with multiple factors, including invasion, circulating pervasion, jump clone and angiogenesis (1). The existing research recognized that such genes as EMMPRIN, MMP-9, TIMP-1 etc. are ones of the primary regulation genes functioning in the invasion and metastasis of lung cancer. The tumor cells destruct the basement membrane on its primary site, go through the interstitial, enter and pierce the blood vessels and being located in the secondary site, which all are associated with the hydrolysis and remodeling of extracellular matrix (ECM) (2). MMP-9 is the main component of ECM degradation, which can degrade almost all ingredients of the ECM, making them play an important role in cell proliferation, tumor invasion and angiogenesis, etc. It’s inhibitor (TIMP-1) can form 1:1 complexes together with MMP-9, inhibiting the MMP-9 roles in lung cancer invasion and metastasis (3). EMMPRIN is an induce factor of MMP-9, and itis closely related with the tumor evolution: it can promote tumor growth by increasing the production quantity of the transparent methane, and can degrade the basement membrane and ECM by stimulating stromal cells and tumor cells to produce a variety of matrix metalloproteinases, thereby to promote the tumor invasion and metastasis (4). For understanding the changes that occur in gene mRNA expression during carcinogenesis may result in improvement of the diagnosis, treatment, and prevention of non-small cell lung carcinoma (NSCLC). We examined, by the way of reverse transcription polymerase chain reaction (RT-PCR), 80 cases of NSCLC patient cancer tissues, 40 cases of cancer adjacent tissues and 10 cases of benign lung tissue in Guangxi, China, to explore the role of the EMMPRIN, MMP-9, TIMP-1 expression in NSCLC incidence and development, which will offer help for the clinical diagnosis, therapy and to predict overall survival in this cohort of patients.
Methods
Materials
We collected, from First Affiliated Hospital of Guangxi Medical University since May, 2007 to October, 2009, 80 resected fresh specimens of NSCLC patients, among these, 51 males and 29 females; age between 40–76 (58.0±6.7) years; 55 cases of ethnic Han, 25 cases of Zhuang; all without any preoperative radiotherapy and chemotherapy. According to 2004 WHO Classifications of Lung Tumor, these specimens were re-classified into 45 cases of squamous carcinoma and 35 cases of adenocarcinoma; and by the differentiation degree, divided into 49 cases of well and medium differentiation, 31 cases of poorly differentiated. According to 1997 Revisions in the International System for Staging Lung Cancer (I–IV), the specimens were divided into 48 cases of early (I–II period), and 32 cases of advanced (III–IV period); 33 cases with lymph node metastasis pathologically confirmed, and 47 cases without lymph node metastasis; 47 cases of long-term smoking (daily smoking >20 cigarettes, over 20 years of continuous smoking, smoking index >400), 33 cases of non-smokers; 40 cases of cancer adjacent tissues (more than 5 cm tumor, the lung tissues without cancer invasion pathologically confirmed) and 10 cases of benign lung tissues were as the control group.
Total RNA extraction and reverse transcription
According to Trizol reagent instructions, total RNA was extracted from the experiment group and the control group. After the reverse transcription reaction referring to M-MLV reverse transcriptase kit instructions, the synthesized cDNA was reserved at −20 °C storage.
PCR reaction
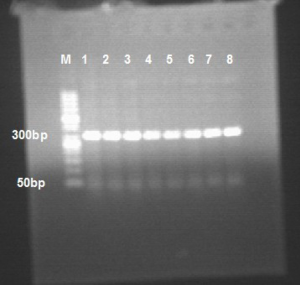
The cDNA as a template, we proceed the amplification according to the appropriate primers and reaction conditions. The upstream primer of EMMPRIN was 5'-GAGAGCAGGTTCTTCGTGAGTTC-3', the downstream primer was 5'-GCCTTTGTCATTCTGGTGCTG-3', and the PCR product was 318 bp. With 3 min at reaction conditions 95 °C, 30 s at 94 °C, 30 s at 60 °C, 30 s at 72 °C, 5 min at 72 °C, we proceed 40 cycles. The upstream primer of MMP-9 was 5'-TTGACAGCGACAAGAAGTGG-3', the downstream primer was 5'-GCCATTCACGTCGTCCTTAT-3', and the PCR product was 179 bp. With 3 min at reaction conditions 94 °C, 45 s at 94 °C, 50 s at 54.5 °C, 60 s at 72 °C, 5 min at 72 °C, we proceed 32 cycles. The upstream primer of TIMP-1 was 5'-GGCATCCTGTTGTTGCTGTGG-3', the downstream primer was 5'-GACGGGACTGGAAGCCCTTTT-3', PCR product was 529 bp. With 3 min at reaction conditions 94 °C, 45 s at 94 °C, 30 s at 54 °C, 60 s at 72 °C, 8 min at 72 °C, we proceed 35 cycles. Reaction system was 25 µL, the reaction products were put in 2% agarose gel for electrophoresis, and observing the target band in gelimaging system. Beijing Nuosai Genome Research Center Co., Ltd. sequenced the reaction products, and the target products were confirmed after comparing the detected sequence in PUB MED.
Statistical
All experimental data applied SPSS 13.0 statistical analysis software for statistical analysis, the test methods used χ2 test, the relationship of EMMPRIN, MMP-9, TIMP-1 expressions in NSCLC tissues was analyzed through the correlation matrix, the Kaplan-Meier method and Log-rank test were used to analyze the correlation of patient survival with gene expression, P<0.05 showed the statistically significant difference.
Results
The expression of EMMPRIN, MMP-9 and TIMP-1 gene: RT-PCR results (Figures 1-3).
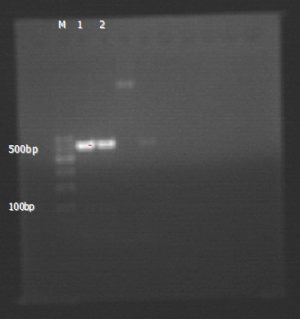
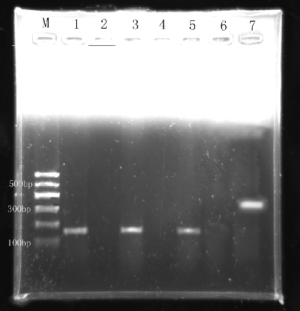
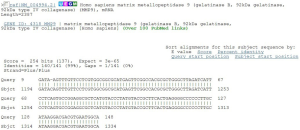
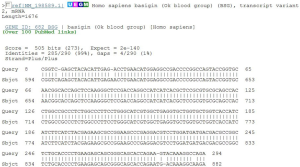
Sequence comparison of PCR results and sequence figures (Figures 4-6).
The EMMPRIN gene expression levels in lung cancer and cancer adjacent lung tissues were 68.7% (53/80), 42.5% (17/40) respectively, the MMP-9 gene expression levels in lung cancer and cancer adjacent lung tissues were 71.25% (57/80), 45.0% (18/40) respectively. The TIMP-1 geneexpression levels in lung cancer and cancer adjacent lung tissues were 50.0% (60/80), 25.0% (10/40) respectively. All three genes had no expression in benign lung tissues. The expression levels of three genes in the lung cancer tissues were higher than in the cancer adjacent lung tissues, and the difference was statistically significant.
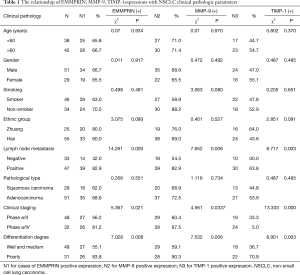
The relationship between EMMPRIN, MMP-9, TIMP-1 expressions and clinical pathologic parameters
The expression of MMP-9 and TIMP-1 genes in NSCLC tissues had no relation with patient age, gender, smoking or not, ethnic group, pathological types (P>0.05), and had relationship with tumor differentiation degree, lymph node metastasis and the clinical staging (P<0.05) (Table 1).
Full table
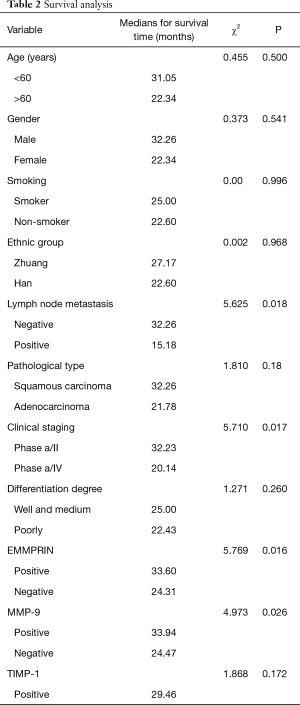
Association between gene expression and survival after surgical resection
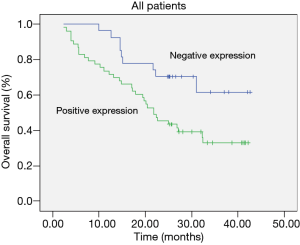
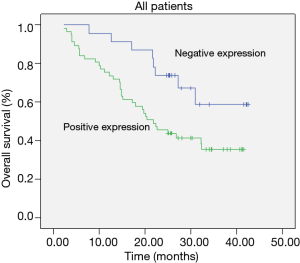
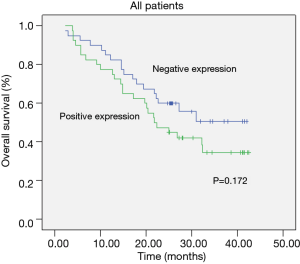
The Kaplan-Meier survival curves in patients positive and negative for EMMPRIN, MMP-9 and TIMP-1 expression. Patients negative for EMMPRIN expression had a significantly longer median overall (more than 33.60 vs. 24.31 months; P=0.016) survival, compared with those positive for EMMPRIN expression. Patients negative for MMP-9 expression had a significantly longer median overall survival (more than 33.94 vs. 24.47 months; P=0.026), than those positive for MMP-9 expression. Patients negative for TIMP-1 expression had a longer median overall survival (more than 29.46 vs. 25.23 months; P=0.172), than those positive for TIMP-1 expression, but the difference was not statistically significant. In this study, TNM stage (P=0.008) and metastasis of lymph node (P=0.007) were also prognostic (Table 2; Figures 7-9).
Full table
The relationship among EMMPRIN, MMP-9 and TIMP-1 expressions
Through the correlation matrix, the result was: through the correlation analysis between EMMPRIN and MMP-9, the contingency coefficient was 0.475 (P<0.05). Through the correlation analysis between EMMPRIN and TIMP-1, the contingency coefficient was 0.473 (P<0.05). And the correlation analysis between MMP-9 and TIMP-1 got the contingency coefficient of 0.454 (P<0.05). It showed that the expressions of EMMPRIN, MMP-9, TIMP-1 in non-small cell lung cancer were positively correlated, and in low positive correlation.
Discussion
The invasion and the metastasis are the most important biological characteristics of malignant tumors, but also the major reasons for patient treatment failure and mortality. Therefore, screening specific tumor-marker is necessary and valuable for early diagnosis and special treatment of tumors. The existing study recognized that, during the lung cancer invasion and metastasis, such genes as EMMPRIN, MMP-9, and TIMP-1 etc. were currently acknowledged as ones of several largest regulatory genes functioning in the invasion and metastasis of lung cancer.
MMP is a group of zinc, calcium-dependent endopeptidases with very similar structure and function, which can almost degrade all protein compositions in ECM. Some studies showed that the degradation of ECM by MMP-9 played a key role in tumor invasion and metastasis, including NSCLC (5). In lung cancer tissues at different staging and classifications, the positive MMP-9 expression was different, the higher staging and the poorer differentiation, the stronger expression (6). Our findings indicate that MMP-9 expression might correlate with OS (P<0.05). The above results suggested that MMP-9 could be used as a marker of the metastatic potential of lung cancer, and the malignancy degree and the metastatic ability of lung cancer could be determined by detecting MMP-9. Foreign literature confirmed this view (7,8). So MMP-9 could be used as the indicator to determine the prognosis of lung cancer patients, and the high MMP-9 expression will indicate a poor prognosis (9,10).
TIMP-1 is the inhibitor of MMP-9, TIMP family is an encoding protein of multi-gene families, which is the specific inhibitor of MMP. TIMP-1, by inhibiting MMP-9, plays the role on anti-tumor growth and preventing the tumor invasion and metastasis (11). In our study, the result showed that the expression rate of TIMP-1 in NSCLC tissues was higher than in cancer adjacent tissues (P<0.05), which kept consistent with Jumper et al. (12) research findings. Even though the negative regulatory role of TIMP-1 on tumor growth was recognized, but TIMP-1 was also highly expressed with increased tumor staging (13-15). The above results suggested that the positive TIMP-1 expression was related with the prognosis of lung cancer patients, patients whose tumors had TIMP-1 expression would have significantly poorer survival (16,17). But our study shows that TIMP-1 expression not correlate to prognosis in NSCLC. It might be caused by the limitation of number of samples (18).
EMMPRIN is an immunoglobulin super family (IgSF) member, which is a transmembrane glycoprotein of about 58 kD molecular weight, as a gene with multiple functions, in a variety of malignant tumors, it not only stimulates the tumor-associated stromal fibroblasts and the endothelial cell to produce MMPS, but also has a significant effect on tumor invasion and metastasis (19-21). In NSCLC tissues, the high EMMPRIN expression inducted the production of MMPs, which led to the degradation of ECM, thereby promoting the invasion and metastasis of cancer cell (22). This study found that the EMMPRIN expression was related with tumor differentiation degree, whether there was lymph node metastasis, TNM staging and other pathological factors, the difference was statistically significant (P<0.05). This study results kept consistent with others research results (23,24). The above results suggested that EMMPRIN might be closely associated with lung cancer invasion and metastasis, it may serve as the important indicator for staging, malignancy degree and prognostic of lung cancer.
The incidence and the development of lung cancer differ from regional distribution and ethnic group, we found that, in the study for EMMPRIN, MMP-9, TIMP-1 in NSCLC tissues of Guangxi Zhuang ethnic, the MMP-9 and TIMP-1 expression rates in Zhuang people were 76% (19/25) and 64% (16/25) respectively, higher than the expression rate of 69% (33/55) and 43% (24/55) in Han people, but the difference was not statistically significant (P>0.05). Chinese Hongyan Wang et al. found that, in their research on the MMP-9, TIMP-1expression in nasopharyngeal carcinoma tissues in Xi’an and Shenzhen regions, the MMP-9, TIMP-1 expression in nasopharyngeal carcinoma tissues of these two regions had no statistically significant difference (P>0.05), our study results were similar with Hongyan Wang (25) research results. In this study, the EMMPRIN expression rate in Guangxi Zhuang people was 80% (20/25), higher than in Han people, but the difference was not statistically significant (P>0.05). It suggested that the expression of EMMPRIN, MMP-9, TIMP-1 in NSCLC had no regional and ethnic differences.
The EMMPRIN expression and the MMP-9 expression were consistent, MMP-9 also showed high expression when EMMPRIN was highly expressed, conversely both showed lower expression, which indicated that EMMPRIN was the upstream factor of MMP-9, being able to stimulate the MMP-9 secretion (26). With further studies in depth, Tang et al. (27) found that the increase in MMP-9 can also promote the soluble EMMPRIN/CD147 expression in tumor matrix. In this study, the expressions of EMMPRIN, MMP-9 and TIMP-1 in NSCLC were positively correlated, and in low positive correlation. It indicated that these three may have mutual induction, synergistic or inhibitory effect in the occurrence and developing process of lung cancer, suggesting that there may be other factors working together, leading to the high expression of these three in lung cancer tissues, but the details need the further study to confirm.
Considering that MMP-9 was the important target for tumor metastasis, vascularization and growth, a number of synthetic MMP inhibitors (MMPls) were born in succession, such as BB-94, Marimastat and Prinomastat etc. But some research results showed that these MMPls were not conducive to the improvement of survival rate, and some were filed to terminate experiments because they shortened survival (28,29). Therefore, researching TIMPS reagents to inhibit tumor invasion and metastasis has become a current hot spot.
In summary, to better to improve the clinical outcome of advanced NSCLC patients, relationship between therapy and prognosis of patients must be investigated. Our findings indicate EMMPRIN and MMP-9 are prognostic factors for overall survival, and are predictive biomarkers in NSCLC patients. Accompanied by enlargement of sample size, TIMP-1 might also be an indicator the above-mentioned. The further research on the role and the co-relationship of EMMPRIN, MMP-9, TIMP-1 will not only help to approach the mechanisms of the NSCLC incidence and development in depth, but also offer help for the gene targeted therapy of NSCLC.
Acknowledgments
Funding: We thank the Guangxi Home Fund (0832011), Guangxi Zhuang Autonomous Region Health Department Issues (Z2008131) and the Guangxi Zhuang Autonomous Region the Large-scale Instrument nets test subsidies.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.12.18). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lemjabbar-Alaoui H, Hassan OU, Yang YW, et al. Lung cancer: Biology and treatment options. Biochim Biophys Acta 2015;1856:189-210. [PubMed]
- Feng D, Xiao J. The relationship of matrix metalloproteinases, their inhibitors and tumor types. Tumor 2009;29:912-5.
- Cai QW, Li J, Li XQ, et al. Expression of STAT3, MMP-1 and TIMP-1 in gastric cancer and correlation with pathological features. Mol Med Rep 2012;5:1438-42. [PubMed]
- Gabison EE, Hoang-Xuan T, Mauviel A, et al. EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie 2005;87:361-8. [Crossref] [PubMed]
- Xue Y, Zhou Q, Zhang S, et al. The expression of the MMP-2, MMP-9 in lung cancer and its relationship with lung cancer metastasis and prognosis. West China Medical Journal 2008;23:225-6.
- Lin SY, Wang YY, Sheu WH. Preoperative plasma concentrations of vascular endothelial growth factor and matrix metalloproteinase 9 are associated with stage progression in papillary thyroid cancer. Clin Endocrinol (Oxf) 2003;58:513-8. [Crossref] [PubMed]
- Herbst RS, Yano S, Kuniyasu H, et al. Differential expression of E-cadherin and type IV collagenase genes predicts outcome in patients with stage I non-small cell lung carcinoma. Clin Cancer Res 2000;6:790-7. [PubMed]
- Boyd S, Tolvanen K, Virolainen S, et al. Differential expression of stromal MMP-1, MMP-9 and TIMP-1 in basal cell carcinomas of immunosuppressed patients and controls. Virchows Arch 2008;452:83-90. [Crossref] [PubMed]
- Zhu Z. Milan criteria and its expansions in liver transplantation for hepatocellular carcinoma. Hepatobiliary Surg Nutr 2016;5:498-502. [Crossref] [PubMed]
- Kamo N, Kaido T, Yagi S, et al. Liver transplantation for small hepatocellular carcinoma. Hepatobiliary Surg Nutr 2016;5:391-8. [Crossref] [PubMed]
- Iniesta P, Morán A, De Juan C, et al. Biological and clinical significance of MMP-2, MMP-9, TIMP-1 and TIMP-2 in non-small cell lung cancer. Oncol Rep 2007;17:217-23. [PubMed]
- Jumper C, Cobos E, Lox C. Determination of the serum matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) in patients with either advanced small-cell lung cancer or non-small-cell lung cancer prior to treatment. Respir Med 2004;98:173-7. [Crossref] [PubMed]
- Nicol GR, Han M, Kim J, et al. Use of an immunoaffinity-mass spectrometry-based approach for the quantification of protein biomarkers from serum samples of lung cancer patients. Mol Cell Proteomics 2008;7:1974-82. [Crossref] [PubMed]
- Ylisirniö S, Höyhtyä M, Turpeenniemi-Hujanen T. Serum matrix metalloproteinases -2, -9 and tissue inhibitors of metalloproteinases -1, -2 in lung cancer--TIMP-1 as a prognostic marker. Anticancer Res 2000;20:1311-6. [PubMed]
- Talvensaari-Mattila A, Pääkkö P, Höyhtyä M, et al. Matrix metalloproteinase-2 immunoreactive protein: a marker of aggressiveness in breast carcinoma. Cancer 1998;83:1153-62. [Crossref] [PubMed]
- Jinga DC, Blidaru A, Condrea I, et al. MMP-9 and MMP-2 gelatinases and TIMP-1 and TIMP-2 inhibitors in breast cancer: correlations with prognostic factors. J Cell Mol Med 2006;10:499-510. [Crossref] [PubMed]
- Pesta M, Kulda V, Kucera R, et al. Prognostic significance of TIMP-1 in non-small cell lung cancer. Anticancer Res 2011;31:4031-8. [PubMed]
- Lee SD, Kim SH. Role of positron emission tomography/computed tomography in living donor liver transplantation for hepatocellular carcinoma. Hepatobiliary Surg Nutr 2016;5:408-14. [Crossref] [PubMed]
- Caudroy S, Polette M, Nawrocki-Raby B, et al. EMMPRIN-mediated MMP regulation in tumor and endothelial cells. Clin Exp Metastasis 2002;19:697-702. [Crossref] [PubMed]
- Tang Y, Nakada MT, Kesavan P, et al. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res 2005;65:3193-9. [Crossref] [PubMed]
- Quemener C, Gabison EE, Naïmi B, et al. Extracellular matrix metalloproteinase inducer up-regulates the urokinase-type plasminogen activator system promoting tumor cell invasion. Cancer Res 2007;67:9-15. [Crossref] [PubMed]
- Yu W, Liu J, Xiong X, et al. Expression of MMP9 and CD147 in invasive squamous cell carcinoma of the uterine cervix and their implication. Pathol Res Pract 2009;205:709-15. [Crossref] [PubMed]
- Rosenthal EL, Shreenivas S, Peters GE, et al. Expression of extracellular matrix metalloprotease inducer in laryngeal squamous cell carcinoma. Laryngoscope 2003;113:1406-10. [Crossref] [PubMed]
- Sienel W, Polzer B, Elshawi K, et al. Cellular localization of EMMPRIN predicts prognosis of patients with operable lung adenocarcinoma independent from MMP-2 and MMP-9. Mod Pathol 2008;21:1130-8. [Crossref] [PubMed]
- Wang H, Jing Y, Zheng J, et al. Expression of MMP-9 and TIMP-1 in nasopharyngeal carcinoma and its signification at Xi’an and Shenzhen. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2010;24:296-8. [PubMed]
- Jouneau S, Khorasani N, DE, Souza P, et al. EMMPRIN (CD147) regulation of MMP-9 in bronchial epithelial cells in COPD. Respirology 2011;16:705-12. [Crossref] [PubMed]
- Tang Y, Kesavan P, Nakada MT, et al. Tumor-stroma interaction: positive feedback regulation of extracellular matrix metalloproteinase inducer (EMMPRIN) expression and matrix metalloproteinase-dependent generation of soluble EMMPRIN. Mol Cancer Res 2004;2:73-80. [PubMed]
- Giavazzi R, Taraboletti G. Preclinical development of metalloproteasis inhibitors in cancer therapy. Crit Rev Oncol Hematol 2001;37:53-60. [Crossref] [PubMed]
- Reckamp KL, Gardner BK, Figlin RA, et al. Tumor response to combination celecoxib and erlotinib therapy in non-small cell lung cancer is associated with a low baseline matrix metalloproteinase-9 and a decline in serum-soluble E-cadherin. J Thorac Oncol 2008;3:117-24. [Crossref] [PubMed]