
Frequencies of actionable mutations and survival in variants of invasive adenocarcinoma of lung
Introduction
Lung cancer is the leading cause of mortality in the world (1). And adenocarcinoma is currently the most common histological type of lung cancer, accounting for more 60% of all non-small cell lung cancer (NSCLC) (2). In 2011, a new pathologic classification of lung adenocarcinoma was redefined by American Thoracic Society and European Respiratory Society (IASLC/ATS/ERS). It was consistent with IALSC/ATS/ERS in the 2015 WHO Classification of Lung Adenocarcinoma in resected specimens (3,4).
Based upon the latest 2015 WHO classification, there are four extremely rare variants of invasive adenocarcinoma of lung (VIA): invasive mucinous adenocarcinoma, colloid adenocarcinoma, fetal adenocarcinoma and enteric adenocarcinoma (4). And the clinico-histological characteristics of this subtype were derived from case reports (5-8). Though widely recognized in lung adenocarcinoma with its abnormal gene spectrum and survival outcomes, little information is known regarding the molecular alterations and prognostic values for VIA.
Here a consecutive cohort of patients with completely resected lung adenocarcinoma were examined for understanding the spectrum of driver genes and assessing its prognosis in VIA subtype according to the 2015 WHO histological classification.
Methods
Patient selection
Between January 2010 and June 2013, a total of 1,120 patients with pathologically confirmed lung adenocarcinoma after complete resection were identified at Zhejiang Cancer institution. Histological typing of lung adenocarcinoma was determined according to the 2015 WHO Classification. Lung cancer staging was performed according to the 7th Tumor-Node-Metastasis (TNM) classification system. The study protocol was approved by ethics committee of Zhejiang Cancer Hospital and all patients provided a written form of informed consent for biomarker analysis (IRB2015-49).
Histological evaluations
Tissue samples were diagnosed by immunohistochemistry. And histological classification was based on the 2015 WHO classification. According to the new criteria, there were four subtypes of invasive mucinous adenocarcinoma, colloid adenocarcinoma, fetal adenocarcinoma and enteric adenocarcinoma. The common immunohistochemical markers were used for all samples, including TTF-1, CK5/6, CK7, CK20, Napsin-A, p40 and p63, etc.
Detection of gene spectrum
Genomic DNA/RNA was extracted according to standard protocols (RNeasy Mini Kit, and QiAamp DNA Mini Kit, Qiagen, Hilden, Germany). Briefly, isolated RNA samples were used for reverse transcription into cDNA using Revert Aid First Strand cDNA Synthesis Kit (Fermentas, St Leon-Rot, Germany). Either genomic DNA or cDNA was used for PCR amplification and sequencing. EGFR, KRAS, NRAS, BRAF, HER2 and PIK3CA were amplified by PCR using genomic DNA. Cyclic sequencing of purified PCR products was performed with PCR primers using ADx Mutation Detection Kit (Amory, Xiamen, China). ALK, ROS1 and RET fusion mRNA were detected by PCR with fusion gene detection kit (Amory, Xiamen, China). Total RNA was extracted with QiagenRNeasy FFPE Kit. And mRNA was reverse-transcribed into cDNA at 42 °C for 1 hour. β-actin was used as an internal control. The specific reverse transcription-polymerase chain reaction (RT-PCR) conditions were as follows: initial denaturation at 95 °C for 5 min, followed by 95 °C for 25 seconds, 64 °C for 20 seconds and 72 °C for 20 seconds to ensure specificity; 31 cycles at 93 °C for 25 seconds, 60 °C for 35 seconds and 72 °C for 20 seconds for data collection and sensitivity analysis. All positive mutation or fusion genes were confirmed with Sanger sequencing. And all experiments were performed according to the user’s manual as described previously (9).
Follow-ups and statistical analyses
All patients were followed up after adjuvant treatment. And recurrence or metastasis was evaluated by thoraco-abdominal computed tomography (CT) and other routine examinations. The last follow-up date was December 31, 2016.
Categorical variables were compared by χ2 test and continuous variables by Mann-Whitney nonparametric test. And Kaplan-Meier method was employed for survival analysis and log-rank for comparison. Overall survival (OS) was defined from the start of confirmed pathology to the date of mortality or the last follow-up. And recurrence-free survival (RFS) was counted as the time from the start of confirmed pathology to recurrence or metastases. Statistical analysis was performed with SPSS 17 software (SPSS, Inc., Chicago, IL, USA).
Results
Clinicopathological characteristics
Among them, 31 patients (2.7%) were enrolled. There were 17 males and 14 females with a median age of 62 years (range, 31–79 years). Twelve patients had a smoking history and 19 had never smoked. The pathological stages were I (n=16, 51.6%), II (n=6, 19.4%) and IIIA (n=9, 29.0%), respectively. According to 2015 WHO Histological Classification criteria, the VIA numbers were as follows: invasive mucinous adenocarcinoma (n=15, 48.4%), enteric adenocarcinoma (n=9, 29.0%), colloid adenocarcinoma (n=4, 12.9%) and fetal adenocarcinoma (n=3, 9.7%). Their clinicopathological characteristics were summarized in Table 1.
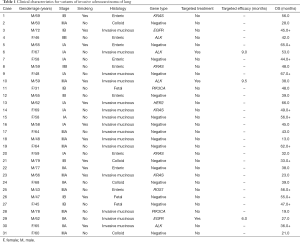
Full table
Genetic results
Fifteen patients harbored gene alterations with a frequency of 48.4% (15/31). The gene alterations included KRAS mutation (n=5, 16.1%), ALK rearrangement (n=4, 12.9%), PIK3CA mutation (n=2, 6.5%), EGFR mutation (n=2, 6.5%), HER2 mutation (n=1, 3.2%) and ROS1 rearrangement (n=1, 3.2%). No mutation of NRAS, BRAF or RET was observed. The frequencies of gene mutations were 60.0%, 55.5%, 0% and 33.3% in invasive mucinous adenocarcinoma, enteric adenocarcinoma, colloid adenocarcinoma and fetal adenocarcinoma respectively.
Comparison of gene spectrum
For comparison, 380 patients with complete resection and pathologically confirmed non-VIA lung adenocarcinoma were randomly selected. And a clinicopathological comparison of patients with VIA and non-VIA was listed in Table 2. No inter-group difference existed in gender, age or smoking status.
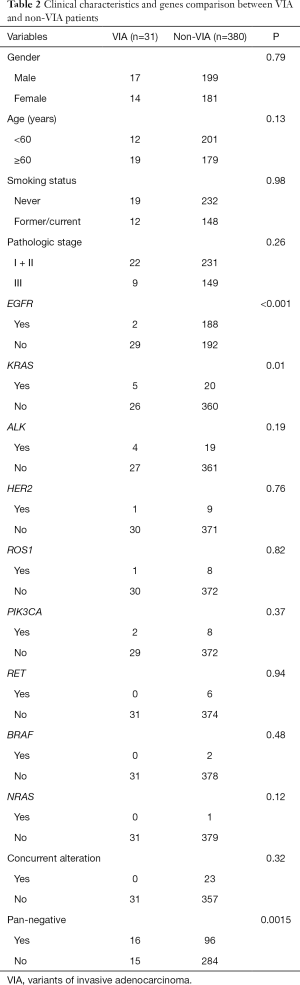
Full table
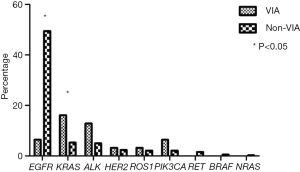
For 380 patients with non-VIA lung adenocarcinoma, all nine gene detections were performed. Gene alterations were detected in 74.7% of non-VIA samples: EGFR mutation (n=188, 49.5%), KRAS (n=20, 5.2%), EML4-ALK rearrangement (n=19, 5.0%), HER2 (n=9, 2.4%), ROS1 rearrangement (n=8, 2.1%), PIK3CA (n=8, 2.1%), RET rearrangement (n=6, 1.6%), BRAF (n=2, 0.5%), NRAS mutations (n=1, 0.3%) and double mutations (n=23, 6.1%). The frequency of gene alterations was higher in non-VIA than VIA patients (74.7% vs. 48.4%, P=0.0015). KRAS mutation was more frequent in VIA than non-VIA patients (16.1% vs. 5.3%, P=0.01) while the pattern reversed in EGFR mutations (6.5% vs. 49.5%, P<0.001). The distributions of gene typing for VIA and non-VIA patients were listed in Table 2 and Figure 1.
Treatments and survival analyses
Among 31 VIA patients, 20 recurred or metastasized and 19 died. Their median RFS and OS were 38.0 (95% CI: 25.8–50.2) and 48.0 months (95% CI: 40.5–55.5) respectively. Targeted therapy was offered for three patients, including EGFR-TKI (n=1) and crizotinib (n=2). The progression-free survival (PFS) was 6.0, 9.0 and 9.5 months respectively. Sixteen patients received first-line chemotherapy with a median PFS of 4.5 months (95% CI: 3.8–5.5).
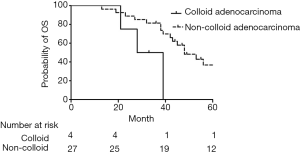
The median OS values were 53.0, 48.0, 28.0 and 48.0 months for invasive mucinous adenocarcinoma, enteric adenocarcinoma, colloid adenocarcinoma and fetal adenocarcinoma respectively. No survival difference existed among different subtypes (P=0.166). However, OS was shorter in colloid adenocarcinoma than other subtypes (48.0 vs. 28.0 months, P=0.034) (Figure 2).
No difference in RFS existed between VIA and non-VIA patients (38.0 vs. 47.0 months, P=0.524). However, there was a trend of worse OS in patients with VIA than those with non-VIA (48.0 vs. 57.0 months, P=0.052) (Figures 3,4).
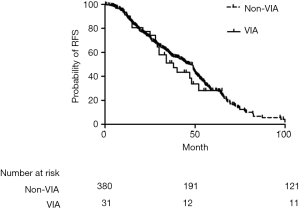
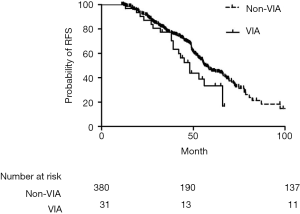
Discussion
As demonstrated here, the frequency of common driver genes of VIA patients was lower than that of non-VIA ones. And there was a trend of shorter OS in VIA patients. Different from non-VIA lung adenocarcinoma, KRAS represented a predominant actionable mutation in VIA patients. To the best of our knowledge, this was the first-ever attempt of detecting the spectrum of driver genes and assessing the prognosis of VIA patients according to the 2015 WHO Pathological Classification of Lung Cancer.
Due to its extreme rarity, VIA has been scantily reported in literature (10). Formerly known as mucinous bronchioloalveolar carcinoma, invasive mucinous adenocarcinoma comprised around half of all VIA patients. In comparison, other subtypes, including enteric adenocarcinoma, colloid adenocarcinoma and fetal adenocarcinoma, were much rarer. In our series, Invasive mucinous adenocarcinoma presented with the highest incidence of 48.4% (15/31) of all tumors, followed by enteric adenocarcinoma, colloid adenocarcinoma and fetal adenocarcinoma. For rarity, the clinicopathology and survival outcome of VIA have not been well documented. In the present study, no significant difference in clinical characteristics was found between VIA and non-VIA patients.
Previous studies demonstrated that the subtypes of NSCLC showed specific driver gene alterations. Over 80% patients with adenocarcinoma harbored definitive molecular genes in East Asian populations (11-14). It is well-known that gene status of EGFR/ALK is associated with the subtypes of adenocarcinoma (15-18). Patients with micropapillary predominant tumors tended to have a high incidence of EGFR mutation. Solid predominant subtype was frequent in ALK rearrangement (15-18). Though as a subtype of lung adenocarcinoma, little was known about its molecular alternations due to the rarity of VIA. Mucinous differentiation was significantly correlated with an absence of EGFR mutation and a presence of KRAS mutation in lung adenocarcinomas with bronchioloalveolar features (19). Similarly, KRAS mutation was a predominant mutation in invasive mucinous adenocarcinoma according to our data. Different from other lung adenocarcinomas, only 13.3% (2/15) patients of invasive mucinous adenocarcinoma harbored EGFR mutations in present study. EGFR mutation was not detected in other 16 patients. Generally, the subtype of VIA was associated with a lower frequency of common driver genes than non-VIA lung adenocarcinoma in current study. Hence, VIA might represent a distinct subset of lung adenocarcinoma.
For a relative rarity of VIA, few studies have explored its prognosis and survival (10,20). In our study, there was no difference in RFS between VIA and non-VIA patients. And a trend of survival difference existed between two groups. And 3/20 of VIA patients received targeted treatment after recurrence while 154/264 non-VIA patients targeted therapy. Treatment imbalance after recurrence might contribute to the discrepancy.
The limitations of our study lied in its retrospective design and fewer samples. Firstly, only 31 patients were enrolled and it could affect clinical and prognosis analysis. Secondly, three patients with gene alternations received targeted treatment after metastasis so that the clinical efficacy of targeted inhibitor was not fully validated. Thirdly, our analysis was limited to a panel of nine genes. And knowledge about other driver events in tumors was rather limited.
Conclusions
In conclusion, the frequency of common driver genes of VIA patients was lower than non-VIA counterparts. KRAS is a predominant actionable mutation and EGFR mutation occurs less frequently in VIA patients. Thus, VIA represents a subset of lung adenocarcinoma with unfavorable survival.
Acknowledgments
Funding: The study was funded by Medical Scientific Research Foundation of Zhejiang Province (Nos. 2015KYA040 &2016KYB046).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.11.19). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Zhejiang Cancer Hospital (IRB2015-49) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Lortet-Tieulent J, Soerjomataram I, Ferlay J, et al. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer 2014;84:13-22. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer, 2015.
- Yousem SA. Pulmonary intestinal-type adenocarcinoma does not show enteric differentiation by immunohistochemical study. Mod Pathol 2005;18:816-21. [Crossref] [PubMed]
- Sato S, Koike T, Yamato Y, et al. Resected well-differentiated fetal pulmonary adenocarcinoma and summary of 25 cases reported in Japan. Jpn J Thorac Cardiovasc Surg 2006;54:539-42. [Crossref] [PubMed]
- Rossi G, Murer B, Cavazza A, et al. Primary mucinous (so-called colloid) carcinomas of the lung: a clinicopathologic and immunohistochemical study with special reference to CDX-2 homeobox gene and MUC2 expression. Am J Surg Pathol 2004;28:442-52. [Crossref] [PubMed]
- Proctor L, Folpe AL, Esper A, et al. Well-differentiated fetal adenocarcinoma of the lung: cytomorphologic features on fine-needle aspiration with emphasis on use of beta-catenin as a useful diagnostic marker. Diagn Cytopathol 2007;35:39-42. [Crossref] [PubMed]
- Lou G, Yu X, Song Z. Molecular Profiling and Survival of Completely Resected Primary Pulmonary Neuroendocrine Carcinoma. Clin Lung Cancer 2017;18:e197-201. [Crossref] [PubMed]
- Ou SH, Kawaguchi T, Soo RA, et al. Rare subtypes of adenocarcinoma of the lung. Expert Rev Anticancer Ther 2011;11:1535-42. [Crossref] [PubMed]
- Zhang Y, Sun Y, Pan Y, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res 2012;18:1947-53. [Crossref] [PubMed]
- Sun Y, Ren Y, Fang Z, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol 2010;28:4616-20. [Crossref] [PubMed]
- Li H, Pan Y, Li Y, et al. Frequency of well-identified oncogenic driver mutations in lung adenocarcinoma of smokers varies with histological subtypes and graduated smoking dose. Lung Cancer 2013;79:8-13. [Crossref] [PubMed]
- An SJ, Chen ZH, Su J, et al. Identification of enriched driver gene alterations in subgroups of non-small cell lung cancer patients based on histology and smoking status. PLoS One 2012;7:e40109 [Crossref] [PubMed]
- Song Z, Zhu H, Guo Z, et al. Correlation of EGFR mutation and predominant histologic subtype according to the new lung adenocarcinoma classification in Chinese patients. Med Oncol 2013;30:645. [Crossref] [PubMed]
- Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 2013;8:52-61. [Crossref] [PubMed]
- Russell PA, Barnett SA, Walkiewicz M, et al. Correlation of mutation status and survival with predominant histologic subtype according to the new IASLC/ATS/ERS lung adenocarcinoma classification in stage III (N2) patients. J Thorac Oncol 2013;8:461-8. [Crossref] [PubMed]
- Revannasiddaiah S, Thakur P, Bhardwaj B, et al. Pulmonary adenocarcinoma: implications of the recent advances in molecular biology, treatment and the IASLC/ATS/ERS classification. J Thorac Dis 2014;6:S502-25. [PubMed]
- Finberg KE, Sequist LV, Joshi VA, et al. Mucinous differentiation correlates with absence of EGFR mutation and presence of KRAS mutation in lung adenocarcinomas with bronchioloalveolar features. J Mol Diagn 2007;9:320-6. [Crossref] [PubMed]
- Sakuma Y, Matsukuma S, Yoshihara M, et al. Distinctive evaluation of nonmucinous and mucinous subtypes of bronchioloalveolar carcinomas in EGFR and K-ras gene-mutation analyses for Japanese lung adenocarcinomas: confirmation of the correlations with histologic subtypes and gene mutations. Am J Clin Pathol 2007;128:100-8. [Crossref] [PubMed]


