
Radiotherapy and radiochemotherapy increase serum levels of pro-inflammatory interleukin-6 and C-reactive protein in patients with head and neck cancers
Introduction
Head and neck cancers arise from the epithelium of the upper aerodigestive tract, and 90% are squamous cell carcinomas (HNSCC). Over 70% of patients are diagnosed with advanced clinical stages III and IV. The current standard treatment is based on surgery and radiotherapy or radiochemotherapy, but the results of treatment remain unsatisfactory. Despite advances in the treatment of HNSCC, the 5-year survival rate remains poor at 50% (1).
Inflammation is associated with many types of cancer including head and neck cancer. In many in vitro and in vivo studies, the proliferative and metastatic capacity of cancer cells is facilitated by inflammatory molecules secreted by the tumour cells themselves as well as by other cells including T and B lymphocytes, monocytes, fibroblasts, keratinocytes, endothelial cells, astrocytes, bone marrow cells, and mesangial cells. The extent of this potential depends mostly on the extracellular environment of the tumour, with intracellular signalling promoted by inflammatory factors including cytokines. Interleukin-6 (IL-6) belongs to the milieu of such cytokines and is high in both tumour tissue and serum. IL-6 regulates such features of cancers as inhibition of apoptosis, promotion of survival, proliferation, angiogenesis, invasiveness, and metastasis. It is also known to regulate cancer metabolism. Moreover, Il-6 protects cancer cells from therapy-induced DNA-damage, oxidative stress, and apoptosis (2-4). Higher expression of IL-6 in tumour cells is therefore associated with poorer responses to radiochemotherapy and an unfavourable prognosis (5). High levels of circulating IL-6 are also associated with poor prognosis, enhanced tumour metastasis, and shorter survival (6,7). IL-6 is the strongest activator of C-reactive protein (CRP) synthesis in the liver. CRP is elevated in chronic inflammation and cancer, and can also serve as prognostic biomarker in head and neck cancers (8).
Besides IL-6 and CRP, transforming growth factor-β (TGF-β) promotes tumour cell proliferation. In normal cells, TGF-β acts as anti-proliferative agent, but in cancer cells the TGF-β signalling pathway becomes mutated, resulting in the loss of its function in these cells. Consequently, TGF-β starts acting on nearby stromal cells, immune cells, and endothelial cells, suppressing the immune response and inducing angiogenesis, thereby promoting the development of cancer (4).
The goal of the study was to investigate the influence of standard therapy (radio- or radiochemotherapy) on serum levels of IL-6, CRP, and TGF-β in patients with head and neck cancers.
Methods
The bioethics committee of the Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology in Warsaw approved the study (No. 44/2012). All participants provided written and informed consent to participate in the study.
Twenty-six patients (n=26) with newly diagnosed and histologically proven head and neck cancer, without distant metastasis, who were admitted to the Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology between 2013 and 2014 were enrolled into this study. All patients underwent a complete medical examination, haematological and biochemical tests, and radiological examinations including magnetic resonance imaging (MRI), chest X-ray, and computed tomography (CT) scans. Clinical and pathological staging was done according to the tumour-node-metastasis (TMN) classification of the International Union Against Cancer (UICC) (9). Patients consisted of women (n=8) and men (n=18), with a mean age of 57 (range, 40–69) years. The majority of patients (n=15) were diagnosed with oropharyngeal cancer. Eleven of them had tumours located in the palatine tonsils, 4 had cancers located at the base of tongue, and 1 cancer was in the soft palate. Nasopharyngeal cancer was diagnosed in 5 patients. Three patients had hypopharynx cancer, and 2 patients had laryngeal cancer. In most cases, the cancers were advanced and categorized as clinical stage III or IVa. The TNM stage at pretreatment evaluations were categorized as follows: clinical T1 (n=4), T2 (n=15), T3 (n=4), and T4 (n=3) by tumour depth and clinical N0 (n=9), N1 (n=5), and N2 (n=12) by lymph node metastasis. None of the patients had distant metastasis.
All patients were referred to radiotherapy alone (n=8) or radiochemotherapy (n=18) with radical intent. Patients referred to radiotherapy alone were treated with an accelerated simultaneous integrated boost (SIB)-intensity-modulated radiation therapy (IMRT) technique. The boost volume was limited to the gross tumour volume (GTV) + 3 mm margin (macroscopic tumour extension was defined on the basis of CT and/or MRI examinations). The dose per fraction given to this volume was 2.25 Gy up to a 67.5-Gy total dose. The planning target volume (PTV)-clinical target volume (CTV) + 3 mm was defined as an area at increased risk of microscopic spread. The dose per fraction given to this volume was 2 Gy up to a total of 60 Gy. The PTV1-ETV (electively irradiated volume) +3 mm received a dose per fraction of 1.8 Gy up to a total of 54–56 Gy. The overall treatment time was 6 weeks (5 fractions per week, 30 fractions). Eighteen patients who were treated with radiochemotherapy received at least two cycles of cisplatin-based chemotherapy at a dose of 100 mg/m2 of body surface area. Patients with nasopharyngeal cancer received three cycles of chemotherapy. Nine patients from this group were irradiated with an IMRT technique, with 2 Gy per fraction up to a total of 70 Gy given in 35 fractions (standard fractionation). The dose per fraction given to the PTV- CTV + 3 mm was 1.8 Gy up to a total of 63 Gy. The remaining 9 patients received radiotherapy delivered with a SIB-IMRT technique as described above. The doses to critical structures were determined by Quantitative Analyses of Normal Tissue Effects in the Clinic (Quantec) recommendations.
The control group consisted of healthy individuals matched with the patient group by age and sex.
Measurements of serum IL-6, TGF-β, and CRP
To measure serum concentrations of IL-6, TGF-β, and CRP, blood samples were collected from each patient before the treatment and at the following time points: 2 weeks after the therapy started, 4 weeks after the therapy started, at the termination of the treatment (6–7 weeks after the therapy stated), and one month post treatment. The design of the experiment allowed us to trace the changes in serum levels of the investigated factors depending on the time from the onset of therapy. A 9-mL volume of blood was drawn in polypropylene tubes and allowed to clot for 30 min before centrifugation. Serum was obtained by centrifugation of the samples for 10 min at approximately 1,000 ×g. The samples were stored at −80 °C before the analysis.
The concentration of cytokines in the serum was determined by enzyme-linked immunosorbent assays (ELISA; R & D Systems tests, Minneapolis, Minnesota, USA). The optical density of each well was determined using a microplate reader (ELx800) set to 450 nm (BIO-TEK Instruments, Inc., Winooski, Vermont, USA). CRP was measured using a turbidimetric analyzer (Cobas), using the test immunoturbidimetric-reinforced latex particles (CRPLX C-reactive Protein Latex, Roche Diagnostics, Indianapolis, Indiana, USA).
Statistical analysis
Data are shown as the mean ± standard deviation (SD). Median values at the investigated time points were compared using the non-parametric Mann-Whitney U test. P values <0.05 were considered to be statistically significant.
Results
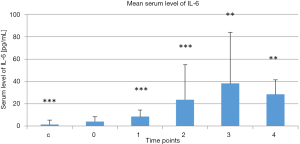
Mean serum levels of IL-6 in the treatment group at the selected time points and in the control group are presented Figure 1. Serum IL-6 levels in the control group were lower than in the treatment group before treatment began (P<0.000). There was a continuous increase in serum IL-6 levels in the treatment group at the first 4 time points versus the baseline level measured before treatment began (P<0.000, P<0.000, P<0.01, and P<0.01, respectively, vs. baseline). Serum IL-6 levels then tended to decrease 1 month post-treatment (4).
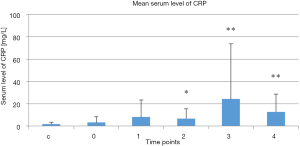
Mean serum CRP levels in the treatment group at the selected time points and in the control group are presented Figure 2. There was no difference in serum CRP levels between the control group and the treatment group at baseline prior to treatment. There was no difference in serum CRP level in treatment group 2 weeks after the therapy started in compare to baseline prior to treatment. However, serum CRP levels increased in the treatment group 4 and 6–7 weeks after the therapy stated and 1 month post-treatment compared to the baseline measurement taken before treatment was started (P=0.02, P=0.001, and P=0.004, respectively, vs. baseline). Serum CRP levels tended to decrease 1 month post-treatment.
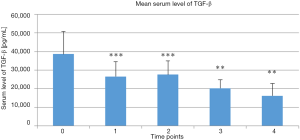
Mean serum TGF-β levels in the treatment group at the selected time points are presented Figure 3. Serum TGF-β levels continuously decreased in the treatment group at the first 4 time points post-treatment versus the baseline value taken before the treatment began (P<0.000, P<0.001, P<0.01, and P<0.01, respectively, vs. baseline).
Discussion
Standard radio- or radiochemotherapy differentially regulated serum levels of IL-6 and TGF-β in patients with head and neck cancers. The basal IL-6 levels in the treatment group were higher than those in healthy individuals in agreement with the results reported by other investigators (10). However, the serum IL-6 level is influenced by many pathological and physiological factors such as acute hyperglycaemia, inflammation, sepsis, a high-fat diet, normal menstrual cycle, stress, or physical exercise, which were not examined in our study (2). The increase in serum IL-6 levels after radio- or radiochemotherapy seemed to be transient. One month after stopping treatment, a trend towards decreases in serum IL-6 levels was observed; however, levels were still higher compare to the pre-treatment baseline level.
A search of the literature revealed that patients with head and neck cancers have a partial TH2 cytokine bias and aberrant cytokine expression. The TH2 phenotype includes IL-6. Cytokine expression is a critical factor for prognosis and sensitivity to antitumor therapy. However, IL-6 exerts its adverse effects mostly via autocrine and paracrine mechanisms, although endocrine adverse effects were also proved (6,11,12). Radiochemotherapy leads to various changes not only in the irradiated area, but in the whole body. The cytokine profile of patients with head and neck cancers receiving radiochemotherapy are more aberrant, and the bias towards the TH2 phenotype is more intensive. An in vitro study conducted on 8 HNSCC cell lines revealed significant increases in cytokine secretion, including IL-6, into the microenvironment of the tumour after sublethal radiation doses, and after treatment with low concentrations of 5-fluorouracil and cisplatin (13).
Cisplatin itself could induce a transient increase in tumorigenic potential in head and neck cancer cells. This effect relies on cisplatin-induced IL-6 expression in these cells, and could compromise the effectiveness of standard therapy (1). Meirovitz et al. showed an increase in serum IL-6 levels in patients with head and neck cancers after radio- and radiochemotherapy, which is in line with our results (14). In our study, serum CRP levels showed similar pattern as IL-6, increasing at the investigated time points in comparison to the pre-treatment level. One month after stopping therapy, the levels tended to decrease as was the case for IL-6. In oesophageal cancers, elevated IL-6 and CRP serum levels after radiochemotherapy were correlated with poor response to radiochemotherapy, and also reflected a poor prognosis. IL-6 was also correlated with greater residual tumour volume (15). Systemic inflammation concurrent with radiochemotherapy indicated by increases in serum IL-6 and CRP is also correlated with other adverse effects from radiochemotherapy including acute mucositis, toxicities, immunological suppression, pain, fatigue, distress, and disturbed sleep (13,16,17).
IL-6 is a pleiotropic cytokine. It has pro-and anti-inflammatory properties and induces the acute-phase reaction. Therefore we cannot exclude also other effects that IL-6 exerts in investigated patients, including the controlling of treatment associated inflammation. However, despite the pleiotropic properties of IL-6, cancer patients with high levels of circulating IL-6 are generally associated with poor prognosis and shorter survival rate, whilst a lower level of IL-6 is associated with better response to therapy (2).
The adverse effects of increased serum IL-6 levels after radiochemotherapy in patients with head and neck cancers could be partially blurred by decreased serum TGF-β levels as we observed at all the investigated time points in our study. In human cancers, TGF-β has a biphasic role in tumour development and progression. Generally, TGF-β suppresses cellular proliferation and promotes cellular differentiation. In head and neck cancers, mutation of type II receptors in the TGF-β signalling pathway is observed and therefore cancer cells are resistant to the proliferation-inhibiting function of TGF-β. While resistant to TGF-β signalling, cancer cells increase the production of this cytokine. As a result, the increase in TGF-β leads to TGF-β-mediated angiogenesis, immune suppression, and stimulation of cell motility (18). In in vitro studies conducted on metastatic and recurrent HNSCC cell lines, increased stage and aggressiveness gave rise to higher TGF-β levels, with the maximum threshold reached at the highest expressing cell lines, which were from stage IV, nonoral, recurrent cancer cell lines. TGF-β activity was 10 times higher in these cell lines than in normal control oral keratinocytes (4). TGF-β signalling in head and neck cancers also enriches cancer stem cell populations, and exogenous treatment with TGF-β induces more resistance to cisplatin treatment (18). Despite that most effects of TGF-β, as with IL-6, depend on paracrine action, the endocrine mode of action cannot be excluded. Therefore, lowering serum TGF-β levels after radiochemotherapy exerts a beneficial effect. It should be always consider, that TGF-β exerts also pleiotropic effects on adaptive immunity: it can suppress adaptive immune responses or can enhance adaptive responses. We cannot exclude such effects in investigated group (19).
Based these results, one should consider implementing anti-IL-6 treatment or blocking its upstream or downstream pathways in patients with head and neck cancers undergoing radiochemotherapy. However, data on the influence of exogenous IL-6 or anti-IL-6R treatment on the effectiveness of cancer therapy is conflicting. Neither exogenous IL-6 nor IL-6R blocking antibodies reversed resistance mediated by IL-6 to cisplatin in cancer cells (1,3). On the contrary, blocking IL-6 signalling in IL-6-rich HNSCC cell lines overcame resistance to erlotinib, a receptor tyrosine kinase inhibitor that acts on the epidermal growth factor receptor (20). Sun et al. revealed that exogenous IL-6 slightly enhanced proliferation but significantly enhanced cell migration and invasion in nasopharyngeal carcinomas, which could be mediated by elevated expression of MMP-2 and -9. This effect of IL-6 was reversed by treatment with a monoclonal anti-human IL-6R antibody (11). Similar results were presented by Chen et al. in pharyngeal cancers (12).
Conclusions
Our results show that radio- and radiochemotherapy are associated with increases in serum IL-6 and CRP level. Both factors could be involved in regulating many tumour-promoting functions and evoking resistance to standard therapy as well as general inflammation-associated symptoms like pain, fatigue, distress, and sleep disturbances. The adverse effects of IL-6 and CRP could be partially blurred by concurrently decreased serum TGF-β levels.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.12.23). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The bioethics committee of the Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology in Warsaw approved the study (No. 44/2012). All participants provided written and informed consent to participate in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Poth KJ, Guminski AD, Thomas GP, et al. Cisplatin treatment induces a transient increase in tumorigenic potential associated with high interleukin-6 expression in head and neck squamous cell carcinoma. Mol Cancer Ther 2010;9:2430-9. [Crossref] [PubMed]
- Kumari N, Dwarakanath BS, Das A, et al. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol 2016;37:11553-72. [Crossref] [PubMed]
- Gao J, Zhao S, Halstensen TS. Increased interleukin-6 expression is associated with poor prognosis and acquired cisplatin resistance in head and neck squamous cell carcinoma. Oncol Rep 2016;35:3265-74. [Crossref] [PubMed]
- Shkeir O, Athanassiou-Papaefthymiou M, Lapadatescu M, et al. In vitro cytokine release profile: predictive value for metastatic potential in head and neck squamous cell carcinomas. Head Neck 2013;35:1542-50. [Crossref] [PubMed]
- Jinno T, Kawano S, Maruse Y, et al. Increased expression of interleukin-6 predicts poor response to chemoradiotherapy and unfavorable prognosis in oral squamous cell carcinoma. Oncol Rep 2015;33:2161-8. [Crossref] [PubMed]
- Ke L, Xiang Y, Xia W, et al. A prognostic model predicts the risk of distant metastasis and death for patients with nasopharyngeal carcinoma based on pre-treatment interleukin 6 and clinical stage. Clin Immunol 2016;164:45-51. [Crossref] [PubMed]
- Yadav A, Kumar B, Datta J, et al. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res 2011;9:1658-67. [Crossref] [PubMed]
- Andersson BÅ, Lewin F, Lundgren J, et al. Plasma tumor necrosis factor-α and C-reactive protein as biomarker for survival in head and neck squamous cell carcinoma. J Cancer Res Clin Oncol 2014;140:515-9. [Crossref] [PubMed]
- Sobin LH, Witedkind CH. UICC TNM classification of malignant tumours, 5-th edition, New York: Wiley-Liss, Inc., 1997:66-9.
- Hoffmann TK, Sonkoly E, Homey B, et al. Aberrant cytokine expression in serum of patients with adenoid cystic carcinoma and squamous cell carcinoma of the head and neck. Head Neck 2007;29:472-8. [Crossref] [PubMed]
- Sun W, Liu DB, Li WW, et al. Interleukin-6 promotes the migration and invasion of nasopharyngeal carcinoma cell lines and upregulates the expression of MMP-2 and MMP-9. Int J Oncol 2014;44:1551-60. [Crossref] [PubMed]
- Chen CC, Chen WC, Lu CH, et al. Significance of interleukin-6 signaling in the resistance of pharyngeal cancer to irradiation and the epidermal growth factor receptor inhibitor. Int J Radiat Oncol Biol Phys 2010;76:1214-24. [Crossref] [PubMed]
- Reers S, Pfannerstill AC, Rades D, et al. Cytokine changes in response to radio-/chemotherapeutic treatment in head and neck cancer. Anticancer Res 2013;33:2481-9. [PubMed]
- Meirovitz A, Kuten M, Billan S, et al. Cytokines levels, severity of acute mucositis and the need of PEG tube installation during chemo-radiation for head and neck cancer--a prospective pilot study. Radiat Oncol 2010;5:16. [Crossref] [PubMed]
- Fujiwara H, Suchi K, Okamura S, et al. Elevated serum CRP levels after induction chemoradiotherapy reflect poor treatment response in association with IL-6 in serum and local tumor site in patients with advanced esophageal cancer. J Surg Oncol 2011;103:62-8. [Crossref] [PubMed]
- Wang XS, Shi Q, Williams LA, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun 2010;24:968-74. [Crossref] [PubMed]
- Sunpaweravong S, Puttawibul P, Ruangsin S, et al. Randomized study of antiinflammatory and immune-modulatory effects of enteral immunonutrition during concurrent chemoradiotherapy for esophageal cancer. Nutr Cancer 2014;66:1-5. [Crossref] [PubMed]
- Bae WJ, Lee SH, Rho YS, et al. Transforming growth factor β1 enhances stemness of head and neck squamous cell carcinoma cells through activation of Wnt signaling. Oncol Lett 2016;12:5315-20. [Crossref] [PubMed]
- Mantel PY, Schmidt-Weber CB. Transforming growth factor-beta: recent advances on its role in immune tolerance. Methods Mol Biol 2011;677:303-38. [Crossref] [PubMed]
- Stanam A, Love-Homan L, Joseph TS, et al. Upregulated interleukin-6 expression contributes to erlotinib resistance in head and neck squamous cell carcinoma. Mol Oncol 2015;9:1371-83. [Crossref] [PubMed]


