
Expression of the desmosome-related molecule periplakin is associated with advanced stage and poor prognosis of esophageal squamous cell carcinoma
Introduction
Esophageal squamous cell carcinoma (ESCC) is an aggressive form of cancer and is one of the most frequent cancers worldwide, particularly in Asian countries (1). Environmental factors, such as smoking and alcohol consumption, are risk factors for ESCC in Western countries (2), whereas the consumption of hot beverages is a major risk factor in Eastern countries (3). Environmental risk factors affect the condition of the epithelial mucosa, inducing mutations and epigenetic changes, which accumulate and induce mucosal dysplasia, and eventually developing into invasive squamous cell carcinoma.
Stratified squamous epithelial cells have many spines that intermingle through dense desmosomes. Periplakin (PPL), a 195-kD membrane-associated protein, is a member of the plakin protein family, which comprises desmoplakin, bullous pemphigoid antigen, and envoplakin (EVPL) (4). Plakin family proteins connect cytoskeletal elements to form desmosomes, intercellular-junction complexes (4,5). PPL expression is observed in keratinized and non-keratinized epithelial cells of the epidermis, urinary bladder, and the oral, esophageal, and cervical mucosae (4). In epidermal epithelial cells, PPL forms stable heterodimers with EVPL. PPL-EVPL heterodimers are localized in desmosomes, the interdesmosomal plasma membrane, intermediate filaments, and the plasma membrane (6).
Although PPL is ubiquitously present in normal squamous cells, PPL-knockout mice do not exhibit obvious abnormalities (7). Furthermore, a past study revealed significant downregulation of PPL in human esophageal cancer tissue and scarce expression in advanced-stage cancers by proteome analyses of cancer tissues compared to adjacent non-cancer tissues (8,9). The decreased expression of PPL was also found in advanced-stage urinary bladder cancer (10). In addition, PPL knockdown is associated with reduced cellular movement and adhesion in pharyngeal cancer cells (11). In a previous study, we determined that PPL is essential for desmosome formation and for the stratified growth of esophageal squamous cells. Although PPL was expressed in all normal esophageal squamous cells, except the Ki67+ basal cell layer, we noticed the presence of PPL-negative cells in ESCC tissues and the proportion of PPL-positive areas in tumors showed considerable variation. We also observed that ESCC cell lines with forced PPL-expression often accumulate into multiple layers, whereas the mock-transfected cells form a monolayer sheet with no stratification (12). In this study, we analyzed the relationships among PPL expression and the clinicopathological features of ESCC tumors. We collected detailed clinical data from 70 patients with ESCC and retrospectively analyzed PPL expression, related clinical features, and ESCC prognosis. Our present study demonstrated that high levels of PPL expression in ESCC are associated with tumor progression, lymph node metastasis, advanced stage cancer, and a poor prognosis. Furthermore, we also observed that increased PPL expression facilitated tumor cell growth in vivo, probably due to PPL-promoted cell-cell adhesion. This mechanism may explain why the PPL-positive group was associated with tumor progression, lymph node metastasis, advanced stage cancer, and a poor prognosis.
Methods
Patients
We enrolled 70 patients with histologically-confirmed ESCC diagnoses. Of these, 46 patients that underwent esophagectomy or endoscopic submucosal dissection from January 2013 to August 2015 at the National Center for Global Health and Medicine (NCGM) provided informed consent before sample collection. This study was approved by the NCGM research ethics committee [121 and 1484]. We also analyzed formalin-fixed, paraffin-embedded sections of surgical specimens, from the remaining 24 patients who were treated between April 2008 and December 2012, by immunohistochemical staining. From these patients, consent was obtained retrospectively in accordance with the dictates of the NCGM research ethics committee [1622].
We retrieved clinicopathological parameters including tumor stage (according to the TNM Classification of Malignant Tumors, 7th edition published by the Union for International Cancer Control) from hospital records. Patients were followed for 1–5 years (until death or until September 7, 2016).
Immunohistochemical analysis
As previously described (12), formalin-fixed paraffin-embedded sections of surgical specimens from ESCC patients were stained with an anti-human PPL antibody (Sigma-Aldrich, Inc., St. Louis, MO, USA), and hematoxylin was used for counterstaining. Antigen retrieval was performed by autoclaving the sections in 10 mM sodium citrate buffer. The ImmPACTTM DAB peroxidase substrate kit (Vector Laboratories, Burlingame, CA, USA) was used for diaminobenzidine staining. For double staining of PPL and Ki67, the MACH2 Double Stain 2 Kit (Biocare Medical, Concord, CA, USA) and the Vulcan Fast Red Chromogen Kit 2 (Biocare Medical) were used to detect signals of anti-Ki67 antibodies (MIB-1, Dako, Glostrup, Denmark). All slides were reviewed by two observers who were blind to clinical and pathological data. Based on the proportion of PPL-positive areas in the malignant tissue, we classified ESCC cases into PPL-negative (the percentage of PPL-positive tumor cells was ≤20% on immunostaining) and PPL-positive groups (the percentage of PPL-positive tumor cells was >20%). Intraobserver reliability of the PPL staining results showed no statistically significant differences. The two observers exhibited high interobserver reliability.
Cell lines and culture
Human ESCC cell line KYSE270 was purchased from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). For passage or to detach cells, we used TrypLE Express (Invitrogen). For the colony formation assays, stably transfected KYSE270/Vector cells and KYSE270/PPL cells (12) (200 cells) were seeded in each well in 24-well plates. After incubation for 3 or 8 days, the colonies were stained with crystal violet. At day 3, four images were captured from each assay and the number of colonies was counted. At day 8, we performed quantification using NIH ImageJ software measuring the area covered with cells as a percentage of the whole captured square area.
Cell growth as subcutaneous xenografts in athymic mice
All experiments were performed according to the Institutional Guidelines for the Care and Use of Laboratory Animals in Research with prior approval from the Animal Experimentation Committee of NCGM [16080]. The male BALB/c nude (nu/nu) mice were purchased from CLEA Japan Inc. (Tokyo, Japan) and maintained under pathogen-free conditions in the NCGM animal facility. Transfection of PPL was prepared as previously described (12). Briefly, human HaloTag® expression vector (pFN21A HaloTag® CMV Flexi® Vector, used for mock-transfection) and human PPL HaloTag® ORF clone in pFN21A (Promega, Madison, WI, USA) were transfected into KYSE270 cells using a lipofectamine LTX reagent (Life Technologies, Inc., Rockville, MD, USA). Stably transfected KYSE270/Vector cells and KYSE270/PPL cells (5.0×106 or 5.0×105) were subcutaneously inoculated in the right and left flanks of 6-week-old BALB/c nude mice, respectively. Tumor size was measured with calipers. Tumor volume was determined by long (a) and short (b) diameters with height (c), calculated as volume = a × b × c ×3.14/6.
Statistical analysis
Each tumor was classified based on its location, size, pathology, the condition of the lymph nodes, and the degree of metastasis (pTNM, 7th edition, 2009). Univariate analyses were performed using one-way ANOVA to compare the expression of PPL for age, the Fisher exact test for gender, tumor location, pT status, pN status, pM status, and disease stage. Survival curves were calculated by the Kaplan-Meier product-limit estimate method and examined using the Log-rank (Mantel-Cox) procedure. Data were expressed as mean ± SD, and results were compared by unpaired Student’s t-tests. Statistical analyses were performed with the Prism 5 statistical program (GraphPad Software, Inc., La Jolla, CA, USA). All tests were two-tailed, and P values <0.05 were considered significant.
Results
Associations between PPL immunoreactivity and ESCC clinicopathological features
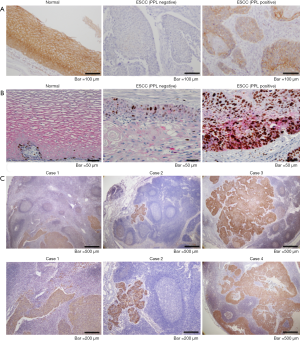
To analyze the relationships among PPL expression and the clinicopathological features of ESCC tumors, the PPL expression levels in tumors was evaluated by immunostaining using formalin-fixed, paraffin-embedded sections from 70 ESCC patients whose demographics are summarized in Table 1. Consistent with other studies (8) and our previous study (12), intense PPL staining was observed at the cell membranes of all of the normal squamous cell epithelium (100% positive) in all cases examined (Figure 1A). On the other hand, half of 70 ESCC tumors were almost totally PPL-negative (Table 1), while others contained more than 40% PPL-expressing cells (Figure 1A). There were clear differences between them. In cases with PPL-positive tumors, localization in tumor cells was not limited to the cell membrane, but was also found in the cytoplasm. Further, PPL-positive cancer cells often expressed Ki67, indicating cell proliferation (Figure 1B). This was in sharp contrast to normal mucosa, where proliferating cells labeled with Ki67 were limited to the basal layer and did not express PPL (Figure 1B). Based on the proportion of PPL-positive areas in the malignant tissue, we classified the ESCC cases as either PPL-negative (the percentage of PPL-positive tumor cells was ≤20% on immunostaining) or PPL-positive (the percentage of PPL-positive tumor cells was >20%). Univariate analysis revealed no differences between the PPL-negative and PPL-positive groups with respect to gender, age, tumor location or distant metastasis. However, we found that relatively high levels of PPL expression in tumors (PPL-positive group) were associated with pT classification (larger primary tumor), pN classification (lymph node metastasis) and advanced stages of cancer (Table 1). PPL was expressed in all of the 26 lymph node metastases analyzed (Figure 1C), indicating that high PPL expression was strongly associated with lymph node metastasis of ESCC. We combined members of the small groups (Table 1) as a large group for comparison to avoid false results. Once again, the PPL-positive group showed correlations with pT classification (T1–2 vs. T3–4) and advanced stages of cancers (I and II vs. III and IV). PPL-positive tumors tended to be associated with lymph node metastases (N0 vs. N+) but this association was not statistically significant (P=0.1513) (Table 2).
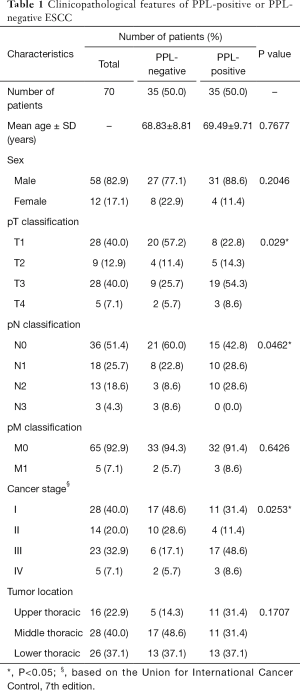
Full table
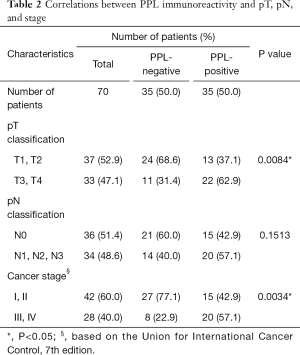
Full table
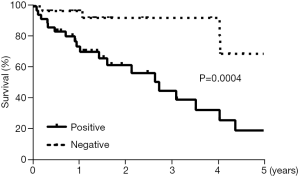
Association between PPL immunoreactivity and a poor prognosis
Patients with tumors that had higher PPL expression had significantly poorer postoperative prognoses than patients with tumors that had lower PPL expression evaluated by immunostaining (Figure 2).
Expression of PPL promoted tumor growth
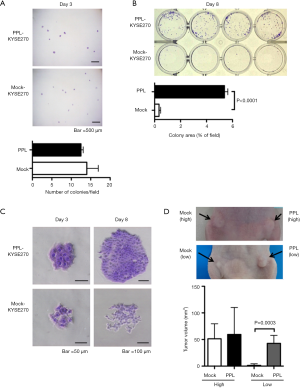
To understand the significance of PPL expression in ESCC, we performed colony formation assay using ESCC cell line KYSE270 cells with induced expression of PPL used in our previous study (12). At day 3, there were no differences in the number of colonies between PPL transfectants and mock transfectants (Figure 3A). However, PPL transfectants formed larger colonies than mock transfectants at day 8 (Figure 3B). We confirmed that PPL transfectants often accumulated and formed stratified layers of ~2–3 cells in colonies, while mock transfectants formed a monolayer sheet with no stratification, consistent with our previous study (Figure 3C). We next tested whether PPL expression affected cell growth in vivo using a xenograft model of tumor formation in BALB/c nude mice. Because the results of colony formation assay seemed to suggest that PPL expression enhanced clonogenicity of ESCC, we inoculated BALB/c nude mice with different number of transfectants. When mice were injected with a relatively large number of cells (5×106 cells), the growth of the PPL transfectants was almost the same as that of the mock transfectants (Figure 3D). In contrast, PPL transfectants grew significantly larger than mock transfectants in vivo when a relatively small number of cells (5×105 cells) were inoculated, indicating that high PPL expression in tumors may promote clonogenicity in vivo, probably due to their capacity to survive and proliferate in an anchorage-independent way by facilitating cell-cell adhesion with desmosome formation. Thus, high PPL expression may eventually promote tumor growth in vivo. This feature may relate to observations of PPL expression and poor prognosis.
Discussion
We previously reported that PPL, a desmosome protein, was downregulated in ESCC and it was associated with relatively high DNA methylation, compared with paired background mucosa. These results suggested that PPL may be methylated and silenced during tumor progression. However, in this study, we analyzed more tumors and unexpectedly found that higher PPL expression in tumors was associated with advanced tumor size, lymph node metastasis, advanced tumor stage, and a poor prognosis. Consistent with our previous study, PPL was expressed in all the normal esophageal squamous cells, except the Ki67+ basal cell layer; however, PPL-negative cells were observed in ESCC tissue samples and the frequency of PPL-positive cells in tumors were less than those in normal mucosa. Further, cytoplasmic localization of PPL was observed in tumors. It has been reported that PPL was cleaved by caspase 6 in keratinocytes after induction of apoptosis (13). Increased expression of caspase 6 in ESCC, compared to expression in normal esophageal mucosa, was also demonstrated (14), which may explain the unusual subcellular localization of PPL in tumors. In vitro expression of PPL has been shown to induce desmosome formation and to facilitate cell-cell adhesion and cell stratification in some ESCC cell lines (12). In this study, we found that ESCC cells with forced expression of PPL gained the ability to form larger colonies than those of mock-transfected cells in vitro. Further, PPL expression accelerated the tumor growth rate in vivo in a xenograft model of tumor formation using BALB/c nude mice. PPL induction promoted ESCC cell growth in vivo, indicating an oncogenic function of PPL. This may appear to conflict with the fact that PPL is generally less expressed in cancer tissues, compared to adjacent normal tissue. As shown in Figure 1B, the critical difference between PPL-expressing cells in normal and malignant tissues is that PPL-positive cells never express a proliferating marker Ki67 in normal mucosa, but often do express it in tumors. When only Ki67+ cells are compared, PPL expression appears highly upregulated in tumors. This indicates that in tumors, the system controlling PPL expression is significantly disturbed. We also speculate that PPL-positive tumors and PPL-negative tumors have different carcinogenic histories. PPL-negative tumors may have developed by losing the differentiating features of squamous cells. Low PPL expression and the lack of a desmosome may limit tumor growth in the normal squamous cell mucosal layer, which has tight cell-cell adhesion, resulting in a propensity towards an intramucosal-type cancer. In contrast, PPL-positive cancer cells may gain the ability to self-proliferate without losing differentiating features, such as the desmosome or matrix adhesion (12). This could promote penetration between squamous cells, further into submucosal layers. This enhanced penetration may be the cause of metastasis and poor prognoses in PPL-positive patients. In this sense, we think it is necessary to separate gene expression changes associated with carcinogenesis (differences between cancerous and normal tissues) from gene expression changes associated with tumor progression (differences related to advanced tumor stages and prognoses).
ESCC is characterized by high malignancy and a poor prognosis. Our studies demonstrate that PPL tumor expression levels could be used as a clinical marker to predict prognoses. Currently, multimodal therapy is performed for ESCC, but, in this study, we have not analyzed the relationships between preoperative chemotherapy or irradiation efficacy and PPL expression. Further studies, including these analyses, as well clarification of the biological roles of PPL in established cancer cells, will refine the value of PPL detection as a clinical prognostic marker of ESCC.
Acknowledgments
We thank Ms. Yasuko Nozaki for her technical assistance, and we thank Drs. Miwa Tamura-Nakano and Chinatsu Oyama of the NCGM EM Support Unit for their technical assistance with the histological analyses.
Funding: This work was supported by JSPS KAKENHI grants numbers JP15K10124, JP15H04503, JP16K09299; grants from the National Center for Global Health and Medicine (25-104, 26-110, 26-117, and 27-1406); and the MEXT-Supported Program for the Strategic Research Foundation at Private Universities.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.01.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the NCGM research ethics committee [121 and 1484]. Informed consent was obtained retrospectively in accordance with the dictates of the NCGM research ethics committee [1622].
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Rossini A, Rapozo DC, Soares Lima SC, et al. Polymorphisms of GSTP1 and GSTT1, but not of CYP2A6, CYP2E1 or GSTM1, modify the risk for esophageal cancer in a western population. Carcinogenesis 2007;28:2537-42. [Crossref] [PubMed]
- Islami F, Boffetta P, Ren JS, et al. High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer 2009;125:491-524. [Crossref] [PubMed]
- Ruhrberg C, Hajibagheri MA, Parry DA, et al. Periplakin, a novel component of cornified envelopes and desmosomes that belongs to the plakin family and forms complexes with envoplakin. J Cell Biol 1997;139:1835-49. [Crossref] [PubMed]
- Sonnenberg A, Liem RK. Plakins in development and disease. Exp Cell Res 2007;313:2189-203. [Crossref] [PubMed]
- DiColandrea T, Karashima T, Määttä A, et al. Subcellular distribution of envoplakin and periplakin: insights into their role as precursors of the epidermal cornified envelope. J Cell Biol 2000;151:573-86. [Crossref] [PubMed]
- Aho S, Li K, Ryoo Y, et al. Periplakin gene targeting reveals a constituent of the cornified cell envelope dispensable for normal mouse development. Mol Cell Biol 2004;24:6410-8. [Crossref] [PubMed]
- Nishimori T, Tomonaga T, Matsushita K, et al. Proteomic analysis of primary esophageal squamous cell carcinoma reveals downregulation of a cell adhesion protein, periplakin. Proteomics 2006;6:1011-8. [Crossref] [PubMed]
- Hatakeyama H, Kondo T, Fujii K, et al. Protein clusters associated with carcinogenesis, histological differentiation and nodal metastasis in esophageal cancer. Proteomics 2006;6:6300-16. [Crossref] [PubMed]
- Matsumoto K, Ikeda M, Sato Y, et al. Loss of periplakin expression is associated with pathological stage and cancer-specific survival in patients with urothelial carcinoma of the urinary bladder. Biomed Res 2014;35:201-6. [Crossref] [PubMed]
- Tonoike Y, Matsushita K, Tomonaga T, et al. Adhesion molecule periplakin is involved in cellular movement and attachment in pharyngeal squamous cancer cells. BMC Cell Biol 2011;12:41. [Crossref] [PubMed]
- Otsubo T, Hagiwara T, Tamura-Nakano M, et al. Aberrant DNA hypermethylation reduces the expression of the desmosome-related molecule periplakin in esophageal squamous cell carcinoma. Cancer Med 2015;4:415-25. [Crossref] [PubMed]
- Kalinin AE, Kalinin AE, Aho M, et al. Breaking the connection: caspase 6 disconnects intermediate filament-binding domain of periplakin from its actin-binding N-terminal region. J Invest Dermatol 2005;124:46-55. [Crossref] [PubMed]
- Oh JE, Kim MS, Kang MR, et al. Expression of apoptosis-related proteins caspase-6, caspase-9, FLIP and BNIP3 in oesophageal squamous cell carcinomas. Pathology 2010;42:492-3. [Crossref] [PubMed]


