
Prophylaxis and treatment of venous thromboembolism in cancer patients: a systemic review and critical appraisal of clinical practice guidelines
Introduction
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is the second leading cause of death in patients with cancer (1). As a major risk factor, patients with cancer have a higher incidence of recurrent VTE and bleeding events from anticoagulation than those without cancer (3-fold and 2.5- to 6-fold, respectively) (2,3). Surgical and medical treatment, catheters, chemo-radiotherapy and infection, all contribute to the thrombosis risk in cancer patients, not merely the malignancy itself (4). Moreover, the development of VTE might lead to a worse survival (5). Though the high incidence rate and negative impact are notorious, VTE is still underestimate by most oncological clinicians.
The increasing number of cancer patients and its negative effect of VTE have challenged the individuals, families and society for the burden of disease and the cost related to the treatment (6). Over the past decade, several national and international organizations have published clinical practice guidelines (CPGs) providing recommendations on the diagnosis and management of VTE in cancer patients to assist clinicians to make appropriate decisions (7). Considering the use of guidelines is crucial to clinical practice, such guidelines as well as the recommendations must be evidence-based to be reliable. However, quality varies among different guidelines and clinicians might confuse the difference in these recommendations. Therefore, it is necessary to assess the methodological quality of these clinical guidelines. Appraisal of Guidelines for Research and Evaluation (AGREE II) instrument was developed by Brouwers et al. and has been widely used in critical appraisal of guidelines (8).
To the best of our knowledge, there was no research published on this issue using AGREE II instrument. In this study, we aimed to access the quality of the existing guidelines related to VTE in cancer patients and to provide a whole picture of the current guideline status.
Methods
Cochrane methodology was applied for systematic reviews in this study (8). Our study included searching for guidelines, applying selection criteria, assessing guideline quality, and collecting relevant content. We defined clinical guidelines as statements that include recommendations intended to optimize patient care according to the definition of guidelines in the Institute of Medicine (IOM) (9).
Search strategies
We conducted a systematic search to search the relevant guidelines on the management of VTE in cancer patients. Information sources included MEDLINE via PubMed and EMBASE. We also obtained additional guidelines by searching the guideline databases and other relevant websites. Major research terms included: guideline, guide, guidance, consensus, recommendation, criteria, statement, VTE and neoplasms (Table S1).
Study selection
The inclusion criteria were developed to select guidelines in our study: (I) the target population included VTE in cancer patients; (II) the guidelines focused on the diagnosis and treatment; (III) the full text available online; (IV) the guidelines in English only. The exclusion criteria were as follow: (I) obsolete guidelines while the new ones updated from the same organization; (II) comprehensive guideline and the study topic only mentioned.
Appraisal of guidelines
We utilized AGREE II instrument and evaluated the selected guidelines. AGREE II consists of 23 items to evaluate six domains of guideline quality, including scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability and editorial independence (10) (details in Table S2). Two appraisers (Qinchang Chen, Qingui Chen) rated each item from one (strongly disagree) to seven (strongly agree) to evaluate an individual domain. When the guidelines reached more criteria or provided greater consideration, the scores increased. In rating process, the reviewers were blinded to each other. After that, if the score for each item in a guideline differed by more than one point, both reviewers explained the reason for the scope and a third reviewer (Huang Kai) would be asked to review the guideline and decide the scope. One reviewer added up all the scores in one domain and calculated each domain score as followed: (obtained score − minimum possible/maximum possible score − minimum possible score)×100%. A guideline was defined as “strongly recommended for use in practice” if four or more than four domains scored over 60%. When most domains scored between 30–60%, a guideline was “recommended for use with some modification”. A guideline was “not recommended for use in practice” if most of the domains were below 30%.
Data collection and recommendations synthesis
A draft data extraction form was developed to collect the document characteristics, including year of publication, country/region, development team, funding organization, recommendations related to the prophylaxis and the treatment for the VTE in cancer patients (Figure S1). Three appraisers abstracted recommendations from selected guidelines on the prevention and treatment of the cancer patients receiving surgical treatment, chemotherapy or central catheter insertion. The use of direct acting oral anticoagulants (DOACs) and the relationship between anticoagulant and cancer survival were also recorded. Among the selected guidelines, we compared the recommendations to identify similarities and difference. The information on prevention and treatment of VTE in cancer patients was only tabulated in Tables 1,2.
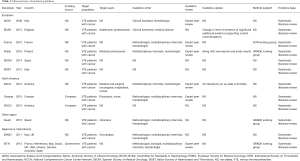
Full table
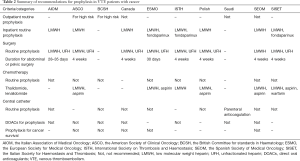
Full table
Results
Search results
A total of 2,335 citations were identified and 2,224 citations were excluded after the review of the titles and abstracts using the general criteria (Figure 1). The full-text review was performed in the remaining 111 citations, among which 98 citations were excluded; 71 citations were not CPGs or consensus statements and 24 were replaced by the updated version while the remaining 4 were due to duplicated publication of the guidelines included. Ultimately, 13 guidelines from 12 national organizations or international working groups were included in this study (11-23). Eight national organizations from Italy (11,23), United States (12,19), United Kingdom (13), Canada (14,15), France (17), Poland (20), Saudi (21), Spain (22) and two regional or international groups (16,18) published these guidelines from 2006 to 2015. All the guidelines included targeted the population of VTE in cancer patients and undertook the process of systematic review. Apart from two guidelines that only included the prophylaxis of VTE (20,23) and two that only included the treatment (17,19), the remaining eight guidelines covered the area of prophylaxis and treatment (11-16,18,22). Two groups reported funding by pharmaceutical company, one by a government institution; the others did not report the funding resource.
Guideline appraisal
The final scores of six domains for every guideline were shown as a percentage in Figure 2. The higher domain score meant the better quality and located the outer perimeter (close to 100%) while the lower domain scores were close to the center (close to 0%). From Figure 2, the American Society of Clinical Oncology (ASCO) guideline, Canada consensus statement and International Society on Thrombosis and Haemostasis (ISTH) guideline had the highest scores in most domains (13,15,16,19). From the threshold of 60% in four or more than four domains to determine the high quality of guideline, AIOM, BCSH and French national guideline were regard as “recommended for use with some modification” while the others were “strongly recommended for use in practice” (12,14,18). There was no guideline defined as “not recommended for use in practice”.
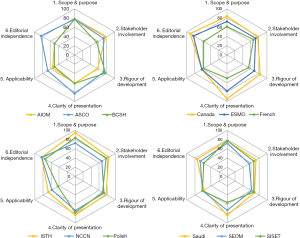
Most guidelines included scored high in domain 1 (scope and purpose) and domain 2 (stakeholder involvement), but scored poorly in domain 5 (applicability). The facilitators and barriers to the application were rarely described in the guidelines included. Most of the guidelines just come up with the recommendations after evidence-based systemic review and did not provide advice or tools to promote the recommendations to be put into practice. Domain 3 (rigor of development) is another area with relatively poor score. Only three guidelines (12,13,20) provided information about guideline update while little statement was found in the remaining documents. Even some of the guidelines included were out of date, which were published for more than 10 years.
Approaches to prophylaxis recommendations
Ten guidance documents covered prophylaxis of VTE in cancer patients. The key recommendations were shown in Table 2. The key areas addressed included the prophylaxis recommendations in outpatients and inpatients, the prophylaxis after receiving surgical treatment, chemotherapy and central catheter insertion. The use of DOACs and the anticoagulation to improve overall survival were also discussed in the guidelines.
For most recommendations in the addressed area, the guidelines included differed somewhat. Routine prophylaxis was recommended for surgical treatment and not recommended for the common chemotherapy, which were the same among the guidelines. Low molecular weight heparin (LMWH) and fondaparinux was recommended in three guidelines for the routine prophylaxis (16,18,23). When receiving abdominal or pelvic surgery, most of the guidelines tended to extend the prophylaxis to 4 weeks. When receiving thalidomide and lenalidomide in the chemotherapy, aspirin was recommended in five guidelines as well as LMWH (12,16,20,22,23). Routine prophylaxis was not recommended for the central catheter in most guidelines but Saudi CPG recommended the parenteral anticoagulation. Outpatient routine prophylaxis was only advocated for the patients with high risk in three guidelines while two did not recommend that in any case (21,22). No guidelines recommended DOACs for prophylaxis and the use of anticoagulation to improve the overall survival in the absence of anticoagulated indicators.
Approaches to treatment recommendations
Ten guidelines provided the recommendations on the treatment for VTE in cancer patients (Table 3). LMWH was still the major choice for the treatment. For long-term treatment of established VTE, there was still controversy about the duration. Four guidelines included (12,13,16,23) were 6 months while five guidelines (11,14,17-19) were 3 months. The higher quality guidelines tended to recommend 3 months. Most of guidelines recommended increasing the international normalized ratio (INR) to the target or switching to the full-dose LMWH. Most of the documents did not recommend the use of DOACs but BCSH guideline recommended that when LMWH impractical. The use of inferior vena cava (IVC) filter was considered in the existence of contraindications or recurrent VTE.

Full table
Discussion
CPGs are widely used in the clinical practice and social health (9,24). The quality of different guidelines varies, and it is commonly believed that making great use of the high quality of the guidelines can improve patient outcome (24). AGREE II instrument has become an accepted standard in the guidelines development and appraisal (10). In this study, we provided an over-view of various guidelines about VTE in cancer patients by the process of systemic review and reported the results of the quality appraisal among the guidelines included. The higher quality helped the clinician to apply more effective recommendations to the patients easily and more patients would benefit from the guidelines with high-quality. Moreover, we also hoped to provide the possible direction to bridge the gap between the guidelines and clinical application.
To the best of our knowledge, this is the first attempt to synthesize and appraise guidelines on the prophylaxis and treatment for the VTE in cancer patients. There were totally 13 guidelines from 12 institutions, which included America, Canada, Italy, Poland, Saudi and so on. According to the GRADE II instrument, of the guidelines included, all were considered “recommendation”; only three guidelines were considered “recommended for use with some modifications” and the remaining guidelines were “strongly recommended for use in practice”, which meant the general quality of the guidelines included was relatively high. However, we also found that the final scores were low at two domains (“rigor of development” and “applicability”), especially in the items including “a procedure for updating the guideline is provided”, “the guideline describes facilitators and barriers to its application” and “the guideline provides advice and/or tools on how the recommendation can be put into practice”. For the 13 guidelines included, update information was only mentioned in three guidelines. The date of publication of five guidelines was more than 5 years old and one was more than 10 years old, which mean that the recent clinical trials with breakthrough in recent years were not included in the document and some guidelines were out of date. Moreover, most guidelines just come up with the recommendations, without the analysis toward the application, such as the cost, life quality and so on. Therefore, the application gap should be bridged in the next generation of guideline.
LMWH was recommended by all the guidelines included as the standard for the prophylaxis and treatment of cancer-associated thrombosis, especially in the long-term treatment of VTE. As shown in Table 3, five guidelines defined the duration as a minimum of 3 months and four defined as 6 months. We observed that the guidelines scored higher tended to recommended 3 months, such as National Comprehensive Cancer Center Network (NCCN), ISTH and Canada consensus statement (14,15,18,19). As a result, 3 months might be the better choice under the result of guideline appraisal. Even though the safety and effectiveness of LMWH have been proved by clinical trials, both clinicians and patients remain reluctant to the established clinical guidelines and only 50% of the patients adhere to use LMWH (25). There are many factors for non-adherence to guidelines. For the long-term treatment, the daily injection will last for at least 3 months, even up to 6 months, which brings a heavy physical and mental burden due to the long-term daily subcutaneous injections (26). Safety concern related to specific condition of cancer patients can also influence the attitude toward the use of LMWH. After receiving the chemotherapy or suffering the hematologic malignancy, the concern about the emergence of thrombocytopenia leads to the positive influence for non-adherence to guidelines (27). The high cost and the life quality are also the important reasons. However, the gap between the guidelines and practice was overlooked by the developers of the guidelines, which results in the low score in the domain 5 (applicability). Indeed, it was not just for scoring high when we appraised the guidelines, but to raise the quality of guidelines effectively to decrease the gap between guidelines and practice because the negative effect of VTE has been overlooked. Thrombosis has been demonstrated to promote the growing, recurrence and metastasis of cancer. The prophylaxis and treatment of VTE have potential benefit for the cancer patients (28). As a result, it is of great importance to seek for an alternative for those clinicians and patients who are reluctant to LMWH.
DOACs including rivaroxaban, apixaban, dabigatran and edoxaban, might be the potential candidate when LMWH is impractical. DOACs not only reduce the cutaneous injection but also have fewer effects on platelet count compared to LMWH. According to the ACCP guidelines, DOAC was priority recommended for the VTE patients without cancer. However, for cancer patients only BCSH guidelines recommend DOACs when LMWH is impractical, due to lack of data for cancer-specific populations (14). However, recent researches have shown that DOACs seem to be as effective and safe as conventional treatment for the prevention of VTE in patients with cancer. Prins et al. observed rivaroxaban group had no statistically difference at the VTE recurrence rate and lower major bleeding rate when compared with LMWH/VKA group (29). The same result was also observed in a meta-analysis including 10 RCTs with 1,132 patients (30). For central catheter-related thrombosis, DOACs were proved to decrease the risk of VTE and to be effective for prophylaxis (31). As a result, DOACs can be considered as an alternative in the patients with active cancer and VTE though more clinical trials are needed.
There were several limitations in our study. First, our assessment was based on the publicly available information from report of guideline organizations. The process of guidelines development might not be reported by the guideline developer, which might include some items in AGREE II criteria and biased the findings. Second, our study only included the CPGs written by English, and those written by other languages might be missed. Finally, the AGREE II instrument only evaluated different items but not the valid content of the recommendations. The quality of the evidence is not assessed. The guidelines might score high due to the use of graded evidence and systematic review, but still make recommendations based on the low-quality evidence. As a result, the actual recommendations only had the strong relationship with evidence used to make them.
Conclusions
The guidelines on VTE in cancer patients were of relatively high quality. More effort is needed to decrease the gap between the practice and guideline. DOACs seem to be potential candidate when clinicians are reluctant to use LMWH, but more researches must be performed.

Full table
Acknowledgments
Funding: The study was supported by the Natural Science Foundation of Guangdong, China (No. 2015A030310346).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.01.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res 2010;125:490-3. [Crossref] [PubMed]
- Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000;160:809-15. [Crossref] [PubMed]
- Schulman S, Zondag M, Linkins L, et al. Recurrent venous thromboembolism in anticoagulated patients with cancer: management and short-term prognosis. J Thromb Haemost 2015;13:1010-8. [Crossref] [PubMed]
- Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008;371:387-94. [Crossref] [PubMed]
- Chew HK, Wun T, Harvey DJ, et al. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol 2007;25:70-6. [Crossref] [PubMed]
- Beckerich F, Hézode C, Robin C, et al. New nucleotide polymerase inhibitors to rapidly permit hematopoietic stem cell donation from a positive HCV-RNA donor. Blood 2014;124:2613-4. [Crossref] [PubMed]
- Wharin C, Tagalakis V. Management of venous thromboembolism in cancer patients and the role of the new oral anticoagulants. Blood Rev 2014;28:1-8. [Crossref] [PubMed]
- Brouwers MC, Kho ME, Browman GP, et al. The Global Rating Scale complements the AGREE II in advancing the quality of practice guidelines. J Clin Epidemiol 2012;65:526-34. [Crossref] [PubMed]
- Vandvik PO, Brandt L, Alonso-Coello P, et al. Creating clinical practice guidelines we can trust, use, and share: a new era is imminent. Chest 2013;144:381-9. [Crossref] [PubMed]
- Dans AL, Dans LF. Appraising a tool for guideline appraisal (the AGREE II instrument). J Clin Epidemiol 2010;63:1281-2. [Crossref] [PubMed]
- Mandalà M, Falanga A, Piccioli A, et al. Venous thromboembolism and cancer: guidelines of the Italian Association of Medical Oncology (AIOM). Crit Rev Oncol Hematol 2006;59:194-204. [Crossref] [PubMed]
- Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American society of clinical oncology clinical practice guideline update 2014. J Clin Oncol 2015;33:654-6. [Crossref] [PubMed]
- Watson HG, Keeling DM, Laffan M, et al. Guideline on aspects of cancer-related venous thrombosis. Br J Haematol 2015;170:640-8. [Crossref] [PubMed]
- Easaw JC, Shea-Budgell MA, Wu CM, et al. Canadian consensus recommendations on the management of venous thromboembolism in patients with cancer. Part 1: prophylaxis. Curr Oncol 2015;22:133-43. [Crossref] [PubMed]
- Easaw JC, Shea-Budgell MA, Wu CM, et al. Canadian consensus recommendations on the management of venous thromboembolism in patients with cancer. Part 2: treatment. Curr Oncol 2015;22:144-55. [Crossref] [PubMed]
- Mandalà M, Falanga A, Roila F. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2011;22:vi85-92. [Crossref] [PubMed]
- Farge D, Bosquet L, Kassab-Chahmi D, et al. 2008 French national guidelines for the treatment of venous thromboembolism in patients with cancer: report from the working group. Crit Rev Oncol Hematol 2010;73:31-46. [Crossref] [PubMed]
- Farge D, Debourdeau P, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost 2013;11:56-70. [Crossref] [PubMed]
- Streiff MB. The National Comprehensive Cancer Center Network (NCCN) guidelines on the management of venous thromboembolism in cancer patients. Thromb Res 2010;125:S128-33. [Crossref] [PubMed]
- Urbanek T, Krasiński Z, Kostrubiec M, et al. Venous thromboembolism prophylaxis in cancer patients—guidelines focus on surgical patients. Acta Angiologica 2016;22:71-102. [Crossref]
- Al-Hameed F, Al-Dorzi HM, AlMomen A, et al. Prophylaxis and treatment of venous thromboembolism in patients with cancer: the Saudi clinical practice guideline. Ann Saudi Med 2015;35:95-106. [Crossref] [PubMed]
- Muñoz Martín AJ, Font Puig C, Navarro Martín LM, et al. Clinical guide SEOM on venous thromboembolism in cancer patients. Clin Transl Oncol 2014;16:1079-90. [Crossref] [PubMed]
- Siragusa S, Armani U, Carpenedo M, et al. Prevention of venous thromboembolism in patients with cancer: guidelines of the Italian Society for Haemostasis and Thrombosis (SISET) (1). Thromb Res 2012;129:e171-6. [Crossref] [PubMed]
- Elwyn G, Quinlan C, Mulley A, et al. Trustworthy guidelines - excellent; customized care tools - even better. BMC Med 2015;13:199. [Crossref] [PubMed]
- Mahé I, Chidiac J, Helfer H, et al. Factors influencing adherence to clinical guidelines in the management of cancer-associated thrombosis. J Thromb Haemost 2016;14:2107-13. [Crossref] [PubMed]
- Debourdeau P, Beckers M, Gerome P, et al. How to improve the implementation of guidelines on cancer-related thrombosis. Expert Rev Anticancer Ther 2011;11:473-83. [Crossref] [PubMed]
- Falanga A, Rickles FR. Management of Thrombohemorrhagic Syndromes (THS) in hematologic malignancies. Hematology Am Soc Hematol Educ Program 2007;165-71. [Crossref] [PubMed]
- Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol 2005;6:401-10. [Crossref] [PubMed]
- Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol 2014;1:e37-46. [Crossref] [PubMed]
- Vedovati MC, Germini F, Agnelli G, et al. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest 2015;147:475-83. [Crossref] [PubMed]
- D'Ambrosio L, Aglietta M, Grignani G. Anticoagulation for central venous catheters in patients with cancer. N Engl J Med 2014;371:1362-3. [Crossref] [PubMed]




