
Beyond chemotherapy: systemic treatment options for hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is a major worldwide health problem. It is the fifth leading diagnosis of cancer, and the second most frequent cause of cancer death in the world, accounting for estimated 782,000 new liver cancer cases and 746,000 cancer deaths (1). While hepatitis B and C are the main worldwide culprits of HCC, alcohol related cirrhosis and NASH cirrhosis are thought to be the major contributors in the United States (2). HCC treatment depends on the size and location of the tumors. If discovered early, curative approaches include resection and liver transplantation. Local ablative procedures such as transarterial chemoembolization and radiofrequency ablation can convert ineligible patients into transplant candidates. Unfortunately, most patients present with advanced disease. In the setting where patients are not candidates for curative therapy or have failed local control approaches, systemic therapy is the next option. In this review, we will briefly review historical systemic options and then focus on sorafenib and the new targeted agents.
Chemotherapy
Single agent chemotherapy
HCC is a chemoresistant tumor. Multidrug resistance protein expression, such as P-glycoprotein and p53, and drug efflux mechanisms render chemotherapeutic agents only minimally effective (3,4). In 1975, Olweny et al. published one of the first studies using single agent doxorubicin in 14 patients with histologically proven HCC (5). The results were promising, with 3 of 11 evaluable patients showing a complete response and an overall 79% response rate. However, subsequent trials failed to show meaningful benefit and also documented significant toxicities from treatment. Even among studies supporting chemotherapy activity, the benefit was of short duration (6,7). Epirubicin and mitoxantrone are other anthracyclines that have been studied, with response rates ranging from 10% to 25% (8-10). Single agent capecitabine, gemcitabine, irinotecan, and others have been used, but the responses were minimal and none provided a survival advantage (10-13) (see Table 1).
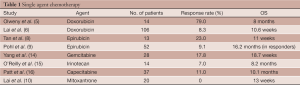
Full table
Combination therapy
Combination chemotherapy regimens have been used with some success. The most well published regimen consists of cisplatin, interferon alpha, doxorubicin, and infusional 5-FU, otherwise known as the PIAF regimen. A phase III randomized, open-label trial included 188 previously untreated patients with histologically confirmed unresectable or metastatic HCC who were randomized to doxorubicin versus PIAF (17). Overall response rate in the doxorubicin group was 10.5% as opposed to 20.9% in the PIAF group (P=0.058). The overall survival in the PIAF group was approximately two months longer (8.67 vs. 6.83 months), but this also was not statistically significant (P=0.83). The toxicity of this regimen is important to note. PIAF produced much more neutropenia (82% vs. 63%, P=0.003), thrombocytopenia (57% vs. 24%, P<0.001), and hypokalemia (7% vs. 0%, P=0.007). Generally, this regimen is not recommended unless the patient has an excellent performance status and can tolerate a rigorous combination regimen.
Combination capecitabine and oxaliplatin (CapeOX) was studied in a single arm phase II trial of 50 previously untreated patients with histologically proven HCC who were not suitable for surgical resection, liver transplantation, or local ablation techniques. As with other agents, the objective response rate was low at 7%, but the disease control rate was 72% with a median duration of 5.4 months (range, 2.2 to 20.5 months). Median overall survival was 9.3 months (18).
Oxaliplatin has also been combined with 5-FU and leucovorin (FOLFOX) in an open label phase III trial randomizing 371 previously untreated advanced HCC patients to FOLFOX versus doxorubicin (19). Initially presented at ASCO 2010, FOLFOX was associated with an increased progression free survival (3 vs. 1.8 months, P<0.01) and median overall survival (6.5 vs. 4.9 months, P=0.07) compared to patients treated with single agent doxorubicin. A 7-month ad-hoc followup analysis showed persistent overall survival trend, however, the study did not achieve its primary overall survival endpoint. Median overall survival for FOLFOX was much lower than reported for sorafenib (Nexavar®, Bayer Pharmaceuticals) in the pivotal SHARP trial (see “sorafenib” section). The authors noted that this trial was designed before definitive sorafenib data was published. Cross study comparison is inherently flawed, but it is important to recognize that SHARP only included 20% of patients with hepatitis B virus (HBV) while this trial had more than 90% of patients with HBV. It is interesting to consider that in the Asian sorafenib trial (see “sorafenib” section), with approximately 70% hepatitis B positive patients, the median OS was exactly the same as this FOLFOX study. Conceivably, this combination is a viable option for patients who may not have ready access to sorafenib.
Finally, combination oxaliplatin and gemcitabine (GEMOX) was studied in advanced HCC with an overall response rate of 19% with 58% having disease stabilization in a phase II trial of 21 HCC patients (20). Other combination cytotoxic regimens include cisplatin and doxorubicin, cisplatin and capecitabine, gemcitabine and cisplatin, and gemcitabine and pegylated liposomal doxorubicin, although it is not clear if any of these regimens confers a survival benefit (21-24) (See Table 2).
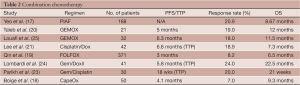
Full table
Targeted therapy
Hepatocarcinogenesis is a complex system of pathways and alterations that has yet to be completely elucidated. What is known about these pathways is that they include growth factors such as epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and insulin like growth factor (IGF). Although these growth factors activate multiple downstream pathways, the RAS/MAPK pathway is important for each one. Activation of RAS/MAPK may lead to HCC growth and proliferation. EGF binds to its cognate receptor, the extracellular domain of epidermal growth factor receptor (EGFR), triggering signal transduction through the RAS/MAPK pathway. VEGF binds to its cognate receptor, VEGFR, promoting HCC angiogenesis. HGF binds to the c-MET receptor, also upstream of the RAS/MAPK pathway. In one particular study, forty percent of patients with HCC were found to express MET and MET inhibition is a promising therapeutic target (26-30). Discussion of all potential pathways is beyond the scope of this review, but we will discuss relevant literature for these important HCC targets.
Anti-VEGF agents
Sorafenib
Elevated expressions of VEGF ligand and receptor have been found in plasma and liver biopsy samples of patients with HCC (31,32). In addition, elevated levels of serum VEGF levels are associated with a worse prognosis (33). For these reasons, targeted VEGF therapies have been a key area of drug development. Sorafenib (Nexavar®, Bayer Pharmaceuticals) changed practice as the first HCC therapy to show a statistically significant and clinically meaningful overall survival benefit. Sorafenib inhibits multiple tyrosine kinases, including VEGFR 1, 2, and 3, targeting angiogenesis pathways. In a phase II trial, 137 patients with advanced HCC treated with sorafenib had a median overall survival of 9.2 months (34). Based on this, Llovet et al. proceeded with the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial, a phase III study that randomized 602 patients with advanced HCC with preserved liver function and no prior systemic treatment to sorafenib versus placebo (35). Patients in the sorafenib arm had a median overall survival of 10.7 vs. 7.9 months in the placebo arm (P<0.001). Although only seven patients (2%) in the sorafenib group experienced a partial response, 204 patients (67%) had disease stability. Patients were primarily from Western countries with Child Pugh A cirrhosis. Approximately 30% of patients had hepatitis C virus (HCV) infection, 20% had HBV infection, and 25% had alcoholic liver disease. While this does not reflect the demographics of HCC worldwide, this is the first agent to consistently show a survival benefit in 30 years of trials. Approved by the Food and Drug Administration in November 2007, sorafenib is now the standard of care for first line systemic treatment in advanced HCC.
To confirm the results of the SHARP trial in a different patient population, sorafenib was studied in a predominantly Asian population. In a phase III trial with inclusion criteria that mirrored the SHARP trial, 229 patients with Child Pugh A cirrhosis were randomized to sorafenib versus placebo (36). The median overall survival in the sorafenib cohort was 6.5 vs. 4.2 months in the placebo arm [hazard ratio (HR) 0.68; 95% CI, 0.50-0.93; P=0.014]. While a statistical significant survival benefit was achieved, the numerical benefit was less than that reported in the SHARP trial despite identical entry criteria and risk stratification. Possible explanations for this include a study population with poorer performance status, more prior therapies, more severe liver disease despite Child Pugh A cirrhosis status, or different disease characteristics (more patients with Hepatitis B) compared to the SHARP trial population.
In both the SHARP trial and the trial by Cheng et al. (36), the entry criteria were limited to patients with Child Pugh A cirrhosis (only about 5% of both trials had Child Pugh B cirrhosis). This excluded a large portion of patients who concominantly have HCC and more advanced liver disease. Presented at ASCO 2013, the results from the Global Investigation of therapeutic Decisions in HCC and of its treatment with sorafeNib (GIDEON) trial (37), suggested that sorafenib can be safely used in patients with Child Pugh Class B cirrhosis. GIDEON was a non-interventional, surveillance trial that observed over 3,000 patients treated with sorafenib and followed for response and adverse events. Interestingly, the rate of drug related adverse events were comparable between Child Pugh A and Child Pugh B liver disease. However, those with more advanced disease as indicated by a worse Child Pugh score had a lower median overall survival.
Sorafenib has been studied in combination with other known active agents. In a randomized phase II trial, patients with inoperable HCC without prior systemic treatment were administered doxorubicin with or without sorafenib (38). Combination doxorubicin and sorafenib improved median time to progression (6.4 vs. 2.8 months, P=0.02) and median overall survival (13.7 vs. 6.4 months; P=0.006) compared to single agent doxorubicin. With the positive results of SHARP, an interim analysis of this trial was conducted, prompting its premature closure due to lack of benefit in the single agent arm. A phase III trial is currently ongoing with combination doxorubicin and sorafenib more appropriately being compared to single agent sorafenib (39).
Sunitinib
With the success of sorafenib, the focus of HCC drug development has now shifted to other molecularly targeted agents. Sunitinib (Sutent®, Pfizer) is a multi-targeted tyrosine kinase inhibitor (TKI) to VEGF 1, 2, and 3. Sunitinib’s antiangiogenic properties suggested activity in HCC and it was evaluated in a single arm phase II study with 37 previously untreated patients with only a partial response in one patient. Its primary endpoint of objective responsive was not met, but there was a stable disease rate of 35% (40). A phase III study that randomized over 1,000 patients to sunitinib versus sorafenib was stopped early due to concerns for futility and safety. At followup, the study actually showed a statistical improvement in median overall survival favoring sorafenib over sunitinib (10 vs. 8.1 months, P=0.0019). Sunitinib was noted to cause more grade 3 and 4 adverse events, occurring in 82% and 73% of patients, respectively (41). Another phase II study was recently reported confirming these phase III results. In 24 patients with advanced HCC without prior systemic therapy, there was a significant worsening of liver functional reserve after sunitinib. Despite a partial response in four patients (12%), grade 3 and 4 adverse events occurred in 80% of patients (42).
Bevacizumab
Bevacizumab (Avastin®, Genentech/Roche), a recombinant humanized monoclonal antibody targeting soluble VEGF-A, was evaluated in 46 HCC patients with Child Pugh A or B cirrhosis and one or less prior systemic therapy who received single agent bevacizumab using doses of 5 and 10 mg/kg every 2 weeks. Of these patients, six had an objective response (13%), including one complete response and five partial responses (43). The median PFS was 6.9 months, median overall survival was 12.4 months, and 53% of the patients were alive at one year. Circulating VEGF levels were decreased from baseline in all patients in the study. With this data, GEMOX and bevacizumab were combined in a phase II trial of 33 patients with unresectable or metastatic HCC who had two or fewer systemic therapies and CLIP score less than three. No patients had a complete response, but six patients had a partial response (20%) and eight patients had stable disease (27%). Median PFS was 5.3 months and overall survival was 9.6 months (44).
Thalidomide
Thalidomide’s (Thalomid®; Celgene Corporation, Warren, NJ) antiangiogenic properties were also explored in HCC, but with disappointing results. In a phase II study of 27 previously treated and untreated patients, one patient had normalization of alpha fetal protein (AFP) and a partial response noted on imaging while two other patients experienced stable disease (45). Another phase II trial enrolled 37 patients, including 13 who had progression after prior therapy, and had similar results with about a 30% stable disease rate while one patient had a partial response (3%) and one patient had a minor response (3%) (46). A phase III trial was opened in 2005, but terminated in 2011 due to lack of patient accrual (47).
EGFR blockade
Erlotinib
Epidermal growth factor receptor (EGFR1) overexpression has been identified in HCC, suggesting that EGFR activation is a potential pathway to HCC development (48). Erlotinib (Tarceva®, Genentech/OSI Pharmaceuticals) is an oral selective TKI of EGFR1, approved for use in non-small-cell lung cancer and advanced pancreatic adenocarcinoma. In a multi-institutional phase II study, erlotinib was administered to 38 patients with surgically unresectable or metastatic HCC, one or fewer prior systemic therapies, and mainly Child Pugh A cirrhosis. Of 34 evaluable patients, 3 (9%) experienced a partial response and 17 (50%) had disease stability. The median overall survival was 13 months, which is superior to historic controls. Grade 3 and 4 adverse events, however, were greater than 60%. Although one of the intended aims of the trial was to stratify response according to the EGFR status, the samples were incomplete and EGFR status was not known in many of these patients (49). Another phase II trial combined erlotinib with bevacizumab for dual EGFR and VEGF blockade. Forty patients with unresectable advanced HCC who had one or fewer prior systemic therapy at a single institution were enrolled, most of whom had Child-Pugh A cirrhosis (85%). Of these patients, 10 (25%) had a partial response and 17 (43%) had stable disease or minor response. The median PFS was nine months and the median overall survival was 15.7 months. For unclear reasons, and in contradiction to the single agent erolotinib data, very few grade 3 or 4 toxicities occurred (50). Combination erlotinib plus bevacizumab has not been compared to sorafenib.
Cetuximab
Cetuximab (Erbitux®, Bristol Meyers Squibb) is a chimeric monoclonal antibody targeting EGFR1 that blocks EGFR dimerization and phosphorylation. It is approved for use in patients with advanced colorectal carcinoma and head and neck tumors. A phase II study examined cetuximab in advanced HCC among 30 patients who had up to two prior systemic therapies (51). No patients achieved an objective response, but five patients (17%) had stable disease with a median duration of 4.2 months. Median PFS was 1.4 months and median overall survival was 9.6 months. EGFR protein expression by immunohistochemistry (IHC) could not be correlated to clinical benefit from cetuximab.
Cetuximab was further investigated in combination with CapOX (52) in 29 patients with Child-Pugh A or B cirrhosis with advanced HCC and no prior systemic therapy. Of 24 evaluable patients, 3 (12.5%) had partial response while 17 (71%) had stable disease, for a disease control rate of 83%. The median progression free survival was 3.3 months, and the median overall survival was 4.4 months, which was quite a bit shorter than using either single agent cetuximab or FOLFOX alone. The reasons for the short TTP, PFS, and OS were not clear.
Lapatinib
Since EFGR1 heterodimerizes with HER2 (EGFR2), dual blockade of these targets was postulated to have efficacy in treating HCC. Lapatinib (Tykerb®, GSK) is an oral irreversible dual inhibitor of EGFR and HER2, currently approved for use in metastatic breast cancer. In a phase II trial, this drug was evaluated in 27 patients with unresectable HCC who had one or less prior systemic therapies (19% had 1 prior therapy). As with many of other EGFR inhibitor trials, lapatinib did not produce any objective responses. However, 10 (40%) patients had stable disease that lasted for over three months in six patients and over one year in two patients. The median PFS was 1.9 months and the median overall survival was 12.6 months. HER2/neu was not overexpressed per fluorescence in situ hybridization (FISH), consistent with other reports that HER2 overexpression in HCC is varied (53).
c-MET blockade and other targeted agents
Tivantinib
Tivantinib is a TKI of c-MET, which can be overexpressed or mutated in many tumor cell types and plays a key role in cell proliferation, survival and metastasis. The c-MET protein is a receptor tyrosine kinase also known as HGF. Overexpression of c-MET portends a worse prognosis in patients with HCC. A randomized phase II trial evaluated 107 previously treated patients and showed a benefit for patients with HCC and a high c-MET expression treated with tivantinib versus placebo in the second line setting. Patients in the treatment arm with high c-MET expression had a significantly increased time to progression (2.9 vs. 1.5 months), progression free survival (2.4 vs. 1.5 months, P=0.01) and disease control rate (50% vs. 20%) (54). Despite a crossover design, a survival benefit trend favored tivantinib. Based on this data, a phase III trial (55) is currently underway, assigning 303 patients to receive tivantinib versus placebo in the second line setting (NCT01755767).
Cabozantinib
Cabozantinib is another promising c-MET inhibitor. It is an oral inhibitor of c-MET and VEGFR2, currently being studied in multiple solid tumors. At ASCO 2012, a phase II trial was presented using cabozantinib in 41 patients with advanced HCC who had no more than one prior systemic treatment. Three patients had a partial response, but 28 patients (78%) had evidence of tumor regression on imaging. As of September 2013, a phase III trial has been opened to further explore the role of this drug in treating HCC (56).
Axitinib
Axitinib (Inlyta®, Pfizer) is a multi-TKI targeting VEGFR 1, 2, 3, PDGFR, and c-Kit. At ASCO GI 2012, interim data from an open-label phase II trial was presented using axitinib in the second-line setting. Data on 15 of the 29 enrolled patients who progressed on prior TKI or anti-VEGF therapy were presented. Of nine patients evaluable for response, there was one partial response with three other patients having tumor shrinkage. Side effects included hypertension, diarrhea, hand foot syndrome, and fatigue. Adverse events required dose reductions in 60% of patients. The full report on this study is pending (57).
Regorafenib
Regorafinib (Stivarga®, Bayer) is a promiscuous multikinase inhibitor with targets including VEGFR2 and 3, Ret, Kit, PDGFR and Raf kinases, approved for metastatic colorectal cancer and metastatic gastrointestinal stromal tumors (GIST). In an open-label phase II trial enrolling 36 patients who progressed on first line sorafenib, the disease control rate was 72% (26 patients), median time to progression was 4.3 months, and median overall survival was 13.8 months. The main toxicities were hand foot syndrome, fatigue, diarrhea, hypothyroidism, and hypertension, with rare grade 3 or 4 adverse events (58). A phase III trial using regorafenib versus placebo in patients who have progressed on sorafenib is ongoing (59).
Linifanib
Linifanib, a TKI of VEGF and PDGFR was studied in a phase II trial involving 44 predominantly Asian patients had up to one prior therapy and demonstrated an objective response rate that was greater than 10% with only mild toxicities (11). In an open-label phase III study, over 1,000 patients were randomized to linifanib versus sorafenib in the first line setting. Patients had advanced HCC, Child Pugh A cirrhosis, and were predominantly Asian. The primary endpoint was overall survival, evaluating both noninferiority and superiority. The median overall survival was 9.1 months compared to 9.8 months on sorafenib, although linifanib had a longer TTP of 5.4 vs. 4.0 months (P=0.001). The overall response rate was 13% in the linifanib arm, however more patients in this arm had dose interruptions and reductions. Thus far, this study has not met its endpoint goals (12).
Brivanib
VEGF and fibroblast growth factor receptor signaling are both implicated in HCC. Brivanib is a selective dual receptor inhibitor of both (13). In a phase III trial of patients who progressed after sorafenib, 395 patients with advanced HCC were randomly assigned in a 2:1 fashion to receive brivanib versus placebo. All patients had previously received sorafenib and the primary endpoint was overall survival. Time to progression (4.2 vs. 2.7 months, P<0.001) and overall response rate (10% vs. 2%, P=0.003) both favored brivanib. However, no difference was found for overall survival (9.4 vs. 8.2 months, P=0.3307), missing the study’s primary endpoint (60).
Brivanib was also studied in the first line setting in a phase III noninferiority trial comparing it to sorafenib among 1,155 patients with advanced HCC who were not eligible for surgical and/or locoregional therapies. The primary endpoint was overall survival, which the study did not meet. Overall median survival was 9.5 months in the brivanib arm versus 9.9 months in the sorafenib arm (HR 1.07, P=0.312). Patients receiving brivanib had a marginally higher objective response rate of 12% vs. 9% compared to the sorafenib arm. Adverse events of any grade were higher in the sorafenib arm, while there were more grade 3 hyponatremia, hypertension, and fatigue in patients receiving brivanib. Unfortunately, brivanib appeared to be less well tolerated than sorafenib with treatment discontinuation due to side effects in 43% of the patients compared to 33% of patients on sorafenib (61). (See Table 3 for targeted agents).
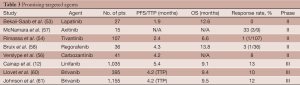
Full table
Discussion
HCC drug development has been marked by a series of disappointing study results. Initial signals of doxorubicin activity over 30 years ago were shattered by the reality of subsequent poor trial outcomes. Since then, therapeutic focus has shifted to targeted therapies that block transduction through the RAS/MAPK pathway known to drive tumorigenesis, including for HCC. Sorafenib, a multi-targeted kinase inhibitor, was the first agent that has consistently demonstrated an overall survival advantage over placebo and other investigational agents, and remains the front-line standard of care for advanced HCC. A variety of reasons can be offered to explain the intransigent nature of HCC. HCC is notorious for tumor heterogeneity introducing the likelihood of resistance. Pathways leading to HCC are also varied, including viral hepatitis, alcohol, and inflammation. In fact, even among patients with viral hepatitis, sorafenib appears to confer a more salutary effect on those without HBV. As has been true with many other historically resistant tumors, enhanced understanding of HCC tumorigenesis pathways holds the promise for finally altering the natural history of this terrible disease. Targeted agents against angiogenesis, and EGFR and c-MET signaling are encouraging first steps. Future research will focus on continued understanding of HCC drivers and combination therapies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Catherine T. Frenette) for the series “Liver Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.12.04). The series “Liver Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Globocan 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009;27:1485-91. [PubMed]
- Soini Y, Virkajarvi N, Raunio H, et al. Expression of p-glycoprotein in hepatocellular carcinoma: a potential marker of prognosis. J Clin Pathol 1996;49:470-3. [PubMed]
- Chenivesse X, Franco D, Bréchot C. MDR1 (multidrug resistance) gene expression in human primary liver cancer and cirrhosis. J Hepatol 1993;18:168-72. [PubMed]
- Olweny CL, Toya T, Katongole-Mbidde E, et al. Treatment of hepatocellular carcinoma with Adriamycin. Preliminary communication. Cancer 1975;36:1250. [PubMed]
- Lai CL, Wu PC, Chan GC, et al. Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma. A prospective randomized trial. Cancer 1988;62:479. [PubMed]
- Nerenstone SR, Ihde DC, Friedman MA. Clinical trials in primary hepatocellular carcinoma: current status and future directions. Cancer Treat Rev 1988;15:1-31. [PubMed]
- Tan YO, Lim F. 4'-Epidoxorubicin (Epirubicin) as a single agent in advanced primary hepatocellular carcinoma--a preliminary experience. Ann Acad Med Singapore 1986;15:169-71. [PubMed]
- Pohl J, Zuna I, Stremmel W, et al. Systemic chemotherapy with epirubicin for treatment of advanced or multifocal hepatocellular carcinoma. Chemotherapy 2001;47:359-65. [PubMed]
- Lai KH, Tsai YT, Lee SD, et al. Phase II study of mitoxantrone in unresectable primary hepatocellular carcinoma following hepatitis B infection. Cancer Chemother Pharmacol 1989;23:54-6. [PubMed]
- Toh HC, Chen PJ, Carr BI, et al. Phase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinoma. Cancer 2013;119:380-7. [PubMed]
- Cainap C, Qin S, Huang WT, et al. Phase III trial of linifanib versus sorafenib in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol 2012;30:abstr 249.
- Huynh H, Ngo VC, Fargnoli J, et al. Brivanib alaninate, a dual inhibitor of vascular endothelial growth factor receptor and fibroblast growth factor receptor tyrosine kinases, induces growth inhibition in mouse models of human hepatocellular carcinoma. Clin Cancer Res 2008;14:6146-53. [PubMed]
- Yang TS, Lin YC, Chen JS, et al. Phase II study of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer 2000;89:750-6. [PubMed]
- O’Reilly EM, Stuart KE, Sanz-Altamira PM, et al. A phase II study of irinotecan in patients with advanced hepatocellular carcinoma. Cancer 2001;91:101-5. [PubMed]
- Patt YZ, Hassan MM, Aguayo A, et al. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer 2004;101:578-86. [PubMed]
- Yeo W, Mok TS, Zee B, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst 2005;97:1532. [PubMed]
- Boige V, Raoul JL, Pignon JP, et al. Multicentre phase II trial of capecitabine plus oxaliplatin (XELOX) in patients with advanced hepatocellular carcinoma: FFCD 03-03 trial. Br J Cancer 2007;97:862-7. [PubMed]
- Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus Doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol 2013;31:3501. [PubMed]
- Taïeb J, Bonyhay L, Golli L, et al. Gemcitabine plus oxaliplatin for patients with advanced hepatocellular carcinoma using two different schedules. Cancer 2003;98:2664-70. [PubMed]
- Lee J, Park JO, Kim WS, et al. Phase II study of doxorubicin and cisplatin in patients with metastatic hepatocellular carcinoma. Cancer Chemother Pharmacol 2004;54:385-90. [PubMed]
- Lee JO, Lee KW, Oh DY, et al. Combination chemotherapy with capecitabine and cisplatin for patients with metastatic hepatocellular carcinoma. Ann Oncol 2009;20:1402-7. [PubMed]
- Parikh PM, Fuloria J, Babu G, et al. A phase II study of gemcitabine and cisplatin in patients with advanced hepatocellular carcinoma. Trop Gastroenterol 2005;26:115. [PubMed]
- Lombardi G, Zustovich F, Farinati F, et al. Pegylated liposomal doxorubicin and gemcitabine in patients with advanced hepatocellular carcinoma: results of a phase 2 study. Cancer 2011;117:125-33. [PubMed]
- Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC): results of a phase II study. Cancer 2007;109:1384-90. [PubMed]
- Ueki T, Fujimoto J, Suzuki T, et al. Expression of hepatocyte growth factor and its receptor c-met proto-oncogene in hepatocellular carcinoma. Hepatology 1997;25:862-6. [PubMed]
- Kaposi-Novak P, Lee JS, Gomez-Quiroz L, et al. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J Clin Invest 2006;116:1582-95. [PubMed]
- Tanabe KK, Lemoine A, Finkelstein DM, et al. Epidermal growth factor gene functional polymorphism and the risk of hepatocellular carcinoma in patients with cirrhosis. JAMA 2008;299:53-60. [PubMed]
- Miura H, Miyazaki T, Kuroda M, et al. Increased expression of vascular endothelial growth factor in human hepatocellular carcinoma. J Hepatol 1997;27:854-61. [PubMed]
- Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 2008;48:1312-27. [PubMed]
- Genesca J, Gonzalez A, Mujal A, et al. Vascular endothelial growth factor levels in liver cirrhosis. Dig Dis Sci 1999;44:1261-2. [PubMed]
- An FQ, Matsuda M, Fujii H, et al. Expression of vascular endothelial growth factor in surgical specimens of hepatocellular carcinoma. J Cancer Res Clin Oncol 2000;126:153-60. [PubMed]
- Poon RT, Lau C, Pang R, et al. High serum vascular endothelial growth factor levels predict poor prognosis after radiofrequency ablation of hepatocellular carcinoma: Importance of tumor biomarker in ablative therapies. Ann Surg Oncol 2007;14:1835-45. [PubMed]
- Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006;24:4293-300. [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in Advanced Hepatocelluar Carcinoma. N Engl J Med 2008;359:378-90. [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomized, double-blind, placebo –controlled trial. Lancet Oncol 2009;10:25-34. [PubMed]
- Marrero JA, Lencioni R, Ye SL, et al. Final analysis of GIDEON (Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma [HCC] and of Its Treatment with Sorafenib [Sor]) in >3000 Sor-treated patients (pts): Clinical findings in pts with liver dysfunction. J Clin Oncol 2013;31:abstr 4126.
- Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma. A randomized trial. JAMA 2010;304:2154-60. [PubMed]
- Available online: http://clinicaltrials.gov/ct2/show/NCT01015833?term=sorafenib+and+doxorubicin+and+hepatocellular+cancer&rank=3
- Faivre S, Raymond E, Boucher E, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicenter, phase II study. Lancet Oncol 2009;10:794-800. [PubMed]
- Cheng A, Kang Y, Lin D, et al. Phase III trial of sunitinib (Su) versus sorafenib (So) in advanced hepatocellular carcinoma (HCC). J Clin Oncol 2011;29:abstr 4000.
- Barone C, Basso M, Biolato M, et al. A phase II study of sunitinib in advanced hepatocellular carcinoma. Dig Liver Dis 2013;45:692-8. [PubMed]
- Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in uresectable hepatocellular carcinoma. J Clin Oncol 2008;26:2992-8. [PubMed]
- Zhu AX, Blaszkowsky LS, Ryan DP, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006;24:1898-903. [PubMed]
- Lin AY, Brophy N, Fisher GA, et al. Phase II study of thalidomide in patients with unresectable hepatocellular carcinoma. Cancer 2005;103:119-25. [PubMed]
- Patt YZ, Hassan MM, Lozano RD, et al. Thalidomide in the treatment of patients with hepatocellular carcinoma: a phase II trial. Cancer 2005;103:749. [PubMed]
- Chen LT. Phase III trial of oral thalidomide in advanced hepatocellular carcinoma with poor liver reserve. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [2013 Sept 9]. Available online: http://clinicaltrials.gov/ct2/show/NCT00225290. NLM Identifier: NCT00225290.
- Hung WC, Chuang LY, Tsai JH, et al. Effects of epidermal growth factor on growth control and signal transduction pathways in different human hepatoma cell lines. Biochem Mol Biol Int 1993;30:319-28. [PubMed]
- Philip PA, Mahoney MR, Allmer C, et al. Phase II Study of Erlotinib (OSI-774) in Patients with Advanced Hepatocellular Cancer. J Clin Oncol 2005;23:6657-63. [PubMed]
- Thomas MB, Morris JS, Chadha R, et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol 2009;27:843-50. [PubMed]
- Zhu AX, Stuart K, Blaszkowsky LS, et al. Phase 2 study of cetuximab in patients with advanced hepatocellular carcinoma. Cancer 2007;110:581-9. [PubMed]
- Sanoff HK, Bernard S, Goldberg RM, et al. Phase II Study of Capecitabine, Oxaliplatin, and Cetuximab for Advanced Hepatocellular Carcinoma. Gastrointest Cancer Res 2011;4:78-83. [PubMed]
- Bekaii-Saab T, Markowitz J, Prescott N, et al. A multi-institutional phase II study of the efficacy and tolerability of lapatinib in patients with advanced hepatocellular carcinomas. Clin Cancer Res 2009;15:5895-901. [PubMed]
- Rimassa L, Porta C, Borbath I, et al. Tivantinib (ARQ 197) versus placebo in patients (Pts) with hepatocellular carcinoma (HCC) who failed one systemic therapy: Results of a randomized controlled phase II trial (RCT). J Clin Oncol 2012;30:abstr 4006.
- Santoro A, Porta C, Rimassa L, et al. Metiv-HCC: A phase III clinical trial evaluating tivantinib (ARQ 197), a MET inhibitor, versus placebo as second-line in patients (pts) with MET-high inoperable hepatocellular carcinoma (HCC). J Clin Oncol 2013;31:abstr TPS4159.
- Cohn AL, Kelley RK, Yang TS, et al. Activity of Cabozantinib (XL184) in Hepatocellular Carcinoma (HCC) Patients: Results From a Phase 2 Randomized Discontinuation Trial (RDT). 2012 ASCO Gastrointestinal Cancers Symposium; January 19-21, 2012; San Francisco, California.
- McNamara MG, Horgan AM, Aspinall A, et al. A phase II trial of second-line axitinib following prior antiangiogenic therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol 2012;30:abstr 314.
- Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur J Cancer 2013;49:3412-9. [PubMed]
- Cheng AL, Finn RS, Kudo M, et al. Regorafenib (REG) in patients with hepatocellular carcinoma (HCC) progressing following sorafenib: An ongoing randomized, double-blind, phase III trial. J Clin Oncol 2013;31:abstr TPS4163.
- Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol 2013;31:3509-16. [PubMed]
- Johnson PJ, Qin SK, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol 2013;31:3517-24. [PubMed]


