
Stereotactic body radiation therapy for primary hepatic malignancies and liver metastases
SBRT in hepatic malignancies
Hepatic malignancies, both primary and metastatic, are increasing in incidence and are associated with significant mortality. Primary hepatic malignancies such as hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (IHC) rank first as the fastest growing cause of cancer death in the United States. The incidence of these diseases has tripled since 1975 (1,2), and the 5-year overall survival rate of primary liver cancer remains dismal at approximately 15% (3). Hepatic metastases from non-liver primaries, such as colorectal (CRC) and breast cancers, are also rapidly rising with approximately 70,000 new cases of CRC liver metastases diagnosed each year. Over the past 40 years, 5-year survival for metastatic CRC has improved from 51% to 65% primarily from improvements in chemotherapy and increased surgical resection of liver metastases (3).
The currently accepted standard of care in hepatic malignancies is surgical resection when feasible. With resection, 5-year survival is approximately 10-50% for HCC and 30-60% for CRC liver metastases (4,5). Unfortunately, less than 30% of hepatic malignancies are resectable at presentation. When resection is not an option in HCC, orthotopic liver transplant (OLT) is the primary curative option. With transplant, 5-year survival increases to 45-80% (6). When patients are not candidates for curative treatment, non-surgical therapies are offered with palliative intent or in the case of HCC, as a possible bridge to OLT. Non-surgical techniques such as transarterial chemoembolization (TACE), radioembolization with Yttrium-90 microspheres, radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), and even high-dose 3-D conformal radiotherapy have been used in this setting. Unfortunately, these non-surgical locoregional therapies are often limited by tumor size, location, number of lesions or degree of hepatic reserve and only TACE and sorafenib have shown a survival benefit in Child-Turcotte-Pugh (CTP) class A patients with HCC in randomized trials (7,8).
Historically, the role of radiation in hepatic malignancies was limited to palliation given the low tolerance of normal hepatic tissue and risk of toxicity. Recently, however, advances in stereotactic radiosurgery (SRS) have led to the application of this technology extracranially. Improvements in immobilization, tumor volume delineation, image guided technology as well as radiation treatment delivery have permitted the use of high doses of radiation to very precise target volumes. Stereotactic body radiation therapy (SBRT) offers several benefits in treating liver malignancies. In addition to being non-invasive, it offers a highly precise mechanism of delivering ablative doses of radiation to tumors while sparing normal or non-tumor hepatic tissue. In sparing normal tissue, toxicity associated with SBRT has been limited (9-12).
In this review, we aim to discuss the current use of SBRT in the management of both primary hepatic malignancies as well as liver metastases.
Patient selection
As with SBRT for other disease sites, SBRT for primary liver tumors and hepatic metastases requires precise and reproducible immobilization. Thus, patients who are unable to tolerate supine positioning or who are unable to lie in an immobilization device for several minutes are poor candidates for SBRT. Also, since SBRT is a highly conformal treatment modality, it is most useful in patients whose liver tumors are readily delineated on MRI or dual phase CT. Regarding HCC, patients with unresectable disease and who are in CTP class A or class B with low CTP scores (<8) have been regarded as the best candidates for SBRT. More detailed criteria for patient selection for SBRT in HCC are informed by previously published phase I prospective trials and RTOG 1112 which is currently open for enrollment, randomizing HCC patients to Sorafenib vs. SBRT followed by Sorafenib (13,14). These criteria are summarized in Table 1. At our center, a distance to critical organs such as adjacent small bowel or stomach of at least 5 mm is essential, but this involves careful planning, image guidance, and dose analysis to not exceed maximum tolerated dose to these structures. In terms of HCC tumor size, Cardenes and colleagues used a tumor diameter of 6 cm as the upper size limit in their phase I trial although the current RTOG 1112 trial allows cumulative tumor size of up to 20 cm (13).

Full table
Selection of patients with metastatic liver lesions for SBRT
Regarding liver metastases, patients considered eligible for SBRT should have a biopsy-proven unresectable metastatic liver malignancy in the presence of adequate hepatic function, and a life expectancy of at least three months (15-18). Typical exclusionary criteria include untreated or uncontrolled primary disease or extensive/widespread metastatic disease. As outlined in further detail below, close attention to the number and size of liver lesions treated with SBRT in the context of the volume and function of the unaffected liver is paramount because of the risk of radiation induced liver disease (RILD). In prospective series of SBRT for liver metastases, a set of criteria almost identical to those used for HCC have been widely implemented to guide patient selection and treatment planning (15-18). The number of tumors to be treated is generally restricted to three or fewer. Similar to studies for HCC, these series have also used a tumor diameter of 6 cm above which SBRT is not recommended. Guidelines for distance to adjacent organs vary widely based on institutional setup. At our institution, a distance between the PTV and adjacent organs of 5 mm or greater is considered acceptable when image guidance is used. Lastly, it is essential to apply a dose constraint to the volume or percent of irradiated normal liver. A common dose/volume constraint for normal liver [total liver minus cumulative gross tumor volume (GTV)] is 700-1,000 cc of normal liver should receive a total dose less than 15 Gy in 3 fractions (15,17,18).
Derivation of liver dose constraint
Dose constraints for liver SBRT are informed by both the surgical literature, which provides insight into the proportion of normal liver which can be safely resected (19), and a conservative conversion from published experiences of conventional fractionation (17). From the surgical literature, it is known that 75-80% of non-cirrhotic liver can be resected safely (19). With the average liver volume being approximately 2,000 cc, one quarter of that is 500 cc. Requiring at least 700 cc of normal/non-cirrhotic liver be spared leaves a volume buffer on average of about 40%. From the conventional fractionation literature, the entire liver has been shown to tolerate at least 33 Gy in 22 fractions (17). The biologically equivalent dose (BED) of this schedule is 49.5 Gy assuming an α/β ratio of three and no repopulation (20). Keeping these assumptions constant, 15 Gy in 3 fractions has a normal tissue BED of 40 Gy, which is less than the expected tissue tolerance observed in conventional fractionation schemes. The maximum total dose to any point in the stomach or small intestine and spinal cord should not exceed 30 and 18 Gy, respectively and the percentage of total kidney to receive a total of 15 Gy assuming 3 fractions should be less than 35% (18).
The risk of developing RILD was further informed by dosimetric studies done by Dawson and colleagues, which revealed a particularly strong correlation of the volume of liver irradiated and mean liver dose to the development of RILD (21,22). From this finding, they developed a method to calculate complication probability factors for non-uniformly irradiated normal liver using dose volume histograms and complication probabilities for uniform partial liver irradiation. In this effective volume (Veff) method, each partial volume element of the histogram is analyzed independently through a power law dose volume relationship (23). With this approach, a non-uniform dose volume histogram is converted to a uniform one with a Veff and a dose equal to the maximum dose to the organ. The complication probability is then obtained from known complication probabilities for uniform partial organ irradiation (21,22).
Technical considerations
SBRT of liver cancer is technically challenging. There is significant inter- and intra-fractional organ motion induced by respiration, and the radiation tolerance of normal liver is low (24,25). The former necessitates the use of larger margin, while the latter discourages it. To make matters worse, liver masses are not typically easy to delineate against the normal liver with in-room cone beam computed tomography (CBCT), leading to uncertainties in image registration and setup (25-29). Since dose-response relationships exist in both primary and metastatic liver cancer, with higher dose resulting in improved outcome, the narrowest possible safety margin is prerequisite in maximizing the therapeutic ratio (30). Consequently, the most accurate and precise target localization technique(s), which minimizes margin size, is essential in liver SBRT. Given the higher doses and tight margins, an effective immobilization and image guidance method is essential to achieve accurate and reproducible treatment delivery. Commonly employed immobilization methods for liver SBRT are synthetic body molds and customized external vacuum cushion bags (18,31). In addition, tumors in the liver may move as much as a few centimeters during the respiratory cycle given the high degree of deformation of the liver. This breathing-related tumor motion can be controlled to a degree and must be measured and accounted for in all stages of treatment preparation (CT simulation and treatment planning) and treatment delivery (7,32,33). Accurate measurement and control of respiratory target motion also allows for reduction of GTV margin expansion to create the PTV. Fiducial markers, small radio-opaque seeds, can be placed with CT guidance prior to simulation and treatment. These can be used for setup verification and to monitor liver motion. In patients previously treated with TACE using the embolic agent Lipiodol, some studies have shown that the embolized area can potentially serve as a direct surrogate for tumor localization on CBCT when combined with active breathing control to minimize setup error and potentially reduce CTV-PTV margins (34,35).
Breathing-related tumor motion can be dampened with active breathing control (i.e., controlled breath hold technique) or abdominal compression. Alternatively, breathing-related tumor motion can be accounted for with respiratory gating or tumor tracking. Published clinical trials of SBRT for HCC and for liver metastases have GTV margin expansions with active breathing control of 5 mm radially and 10 mm cranio-caudally. With abdominal compression, volume expansions were 7 mm radially and 15 mm cranio-caudally (13,14,18). When respiratory gating or 4D-CT is used, an internal tumor volume (ITV) is created to define the target volume in which the GTV includes the tumor position in all phases of the respiratory cycle. A small margin is added to the ITV to create a PTV accounting for daily setup variation. Representative SBRT treatment plans for a patient with HCC and for a patient with a metastasis to the liver are depicted in Figures 1 and 2, respectively.
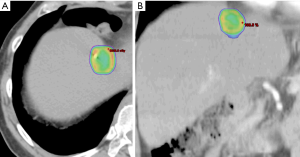
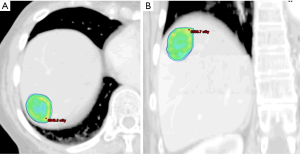
As stated, the use of stereotactic body frames, active breathing control, and abdominal compression plates have been popular in limiting most diaphragm motion to less than 10 mm (36-41). Even with reduced motion, however, the problem with image registration uncertainty still remains. An effective solution to this lack of soft tissue contrast is the use of percutaneously inserted fiducial markers as a surrogate (42-46). This approach is quite effective because the metal markers are radio-opaque and are thus readily visible in X-ray projections. Therefore, using markers to characterize the daily liver motion and subsequently adjusting the treatment setup is an effective strategy to increase treatment accuracy. At our institution, this has been the regular practice. We use three implanted fiducials placed within 3 cm of the tumor edge but not in the tumor itself to avoid metal induced artifacts in CT and possible spreading of the cancerous cells during its insertion. At the treatment table, we employ kilovoltage (kV) X-ray imaging, CBCT, and kV fluoroscopic imaging in sequence to assess the respiratory-induced liver motion as well as to make adjustments based on their movement characteristics. We recently analyzed the motion characteristics of twenty liver SBRT patients (26). The motion trajectories of the implanted fiducials are reconstructed and nicely visualized in Figure 3.
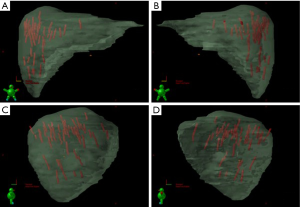
Real-time tumor tracking is another method of accounting for respiratory motion employed by the Cyberknife® system and Novalis ExacTrac® patient positioning system known as Brain-LAB (ExactTrac; BrainLab Inc, Westchester, IL). The Cyberknife® system (Accuray Inc, Sunnyvale, CA) requires the placement of 3 to 6 internal fiducial makers 3 to 5 mm in size. A 3-5 mm GTV expansion for liver tumors is typical in series using Cyberknife® (47). In the Cyberknife® system, a kV camera mounted on the robotic arm alongside the linear accelerator performs real-time fiducial tracking and respiratory motion modeling. For institutions using the Brain-LAB patient positioning system, external body fiducial markers are monitored from ceiling-mounted infrared cameras and respiratory gating is obtained using a relaxed, end-expiratory breath-hold technique.
Review of SBRT for hepatic malignancies
One of the earliest studies to explore the use of SBRT in hepatic malignancies for inoperable or non-surgical patients was published by Blomgren et al. in 1995. The study included 42 tumors in 31 patients with solitary hepatic, lung or retroperitoneal tumors that ranged in size from 2 to 622 cm3, with a mean volume of 78 cm3. Using total mean doses from 8-66 Gy with a mean dose of 41 Gy, they reported a progression free survival of 80% over a period of 1.5-38 months. Additionally, 50% of the tumors showed a reduction in size or disappeared (48).
Following this initial study, a phase I/II dose-escalation trial was conducted by Herfarth et al. using single-dose SBRT for inoperable hepatic malignancies. Thirty-five patients with 55 tumors, including both primary and metastatic lesions, were treated to doses between 14 and 26 Gy in a single fraction. Size ranged from 1 to 132 cm3, with a median size of 10 cm3. After 6-weeks of follow-up, 54 (98%) of the tumors were locally controlled. Local control rate was reported as 81% at a median follow-up of 18 months after accounting for dose-escalation and learning phase (9).
In a retrospective study by Wulf et al. involving both primary liver tumors and hepatic metastases, higher dose regimens were found to significantly improve local control. Five patients with primary hepatic malignancies and 39 patients with 51 liver metastases were included. Using “low-dose” regimens of 3×10 and 4×7 Gy, they reported actuarial local control rates of 86% and 58% at 12 and 24 months, respectively. “High-dose” regimens of 3×12-3×12.5 and 1×26 Gy resulted in local control rates of 100% and 82% at 12 and 24 months, respectively. At a median follow-up of 15 months, all primary liver malignancies were controlled, whereas nine local failures were seen in the hepatic metastases group (12).
A prospective, phase I-II trial by Méndez Romero et al. involving 45 unresectable hepatic lesions, both primary and metastatic, showed local control rates of 94% and 82% at 1 and 2 years, respectively with a median follow-up of 12.9 months. Dose was adjusted for larger lesions or the presence of cirrhosis. Most lesions received 3 fractions of 12.5 Gy, however, lesions ≥4 cm or HCC with cirrhosis were treated to lower doses or with more extended schedules, such as 5 5-Gy fractions or 3 10-Gy fractions. This trial found toxicity to be greater in patients with more severe liver disease, such as patients with CTP-B liver disease (49).
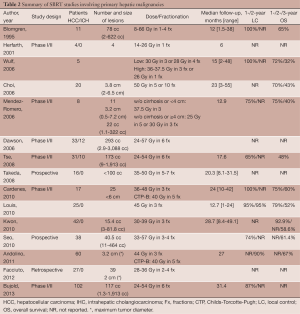
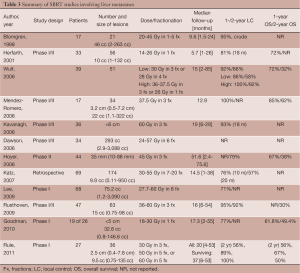
These early studies showed that SBRT is an effective, safe, and feasible option in the local control of unresectable hepatic malignancies. However, there is little consensus on dosing and fractionation schedules among the studies. Several recent prospective studies have aimed to address these issues while selectively limiting their observations to either primary liver malignancies (Table 2) or hepatic metastases (Table 3). Because the quality and functioning of the non-tumor liver parenchyma becomes important in determining maximum tolerable dose and because it varies between primary and metastatic hepatic malignancies, it is helpful to discuss target volumes, dosing and fractionation individually.
Full table
Full table
Primary hepatic malignancies
In a small study by Choi et al. involving 20 patients with small (2-6.5 cm), inoperable HCCs in the setting of CTP-A or -B class liver disease, overall response rate was reported as 80% at a median follow-up of 23 months using 50 Gy in 5 or 10 fractions. One and 2-year survival rates were 70% and 43%, respectively with a median survival of 20 months. Similarly, 1- and 2-year disease free survival rates were 65% and 32.5%, respectively with a median disease free survival of 19 months (50).
Similarly, Tse et al. conducted a phase I trial involving 41 patients with unresectable primary hepatic malignancies, 31 CTP-A HCCs and 10 IHCs. Dose was adjusted to reflect the volume of liver irradiated, taking into account the estimated risk of liver toxicity. Lesions were larger, ranging in size from 9 to 1,913 mL with a median size of 173 mL. Patients were treated to doses between 24 and 54 Gy with a median dose of 36 Gy in 6 fractions over two weeks. Median survival was 11.7 months for HCC patients and 15 months for patients with IHC (14).
In a prospective, single institution study by Takeda et al., 16 patients with small (<100 cm3), solitary HCCs were treated to 35-50 Gy in 5-7 fractions over 5-9 days. With a median follow-up of 611 days, 15 of the 16 patients showed either a complete response (CR) or stable disease. Six of the patients developed intrahepatic recurrences outside of the treated volume (51).
At Indiana University, Cardenes et al. conducted a phase I dose escalation trial using SBRT for primary HCC in unresectable, CTP-A or -B patients with 1-3 lesions. Seventeen patients with 25 lesions were included. Dose was escalated from 36 to 48 Gy in 3 fractions for patients with CTP-A class disease. During the study, patients with CTP-B disease developed significant toxicity at 3×14 Gy. The protocol for CTP-B disease was then amended, extending the fractionation schedule to 40 Gy in 5 fractions. At a median follow-up of 24 months, local control and stabilization of disease were reported as 100%. Overall survival at 1- and 2-year was 75% and 60%, respectively (13).
More recently, a series by Louis et al. included 25 patients with HCC and CTP-A or -B liver disease who were either unresectable or ineligible for other treatment modalities. Using SBRT delivered with Cyberknife® system, lesions were treated to 45 Gy in 3 fractions over 10-12 days. At a median follow-up of 12.7 months, six patients had died. Actuarial local control at 1- and 2-years was reported as 95% and 1- and 2-year overall survival was 79% and 52%, respectively (52).
In 2010, Kwon et al. reported on long-term effects of SBRT for HCC lesions that were ineligible for locoregional therapies or unresectable. Forty-two patients with small (≤100 cc, median volume 15.4 cc) HCCs were treated to 30-39 Gy in 3 fractions. At a median follow-up of 28.7 months, 86% of patients experienced either a complete or partial response, with most achieving a CR. Smaller tumors (<32 cc) had significantly better in-field progression free survival and overall survival. In-field progression free survival at 1 and 3 years was 72% and 67.5%, respectively. Overall 1- and 3-year survival rates were 92.9% and 58.6%, respectively (53).
Similarly, a Korean, prospective trial by Seo et al. evaluated SBRT as a salvage therapy for inoperable HCC (<10 cm) following hepatic TACE. The study included 38 patients treated with SBRT to 33-57 Gy in 3-4 fractions. Doses were adjusted for tumor volume with most tumors ranging in size from 11 to 464 cc. At 2-years, overall survival was reported as 61% and progression-free survival was 66%. Univariate analysis showed ITV <100 cc and SBRT doses <42 Gy in 3 fractions to be significant prognostic factors of overall survival; whereas, multivariate analysis identified SBRT dose as the only prognostic factor (54).
In another study at Indiana University by Andolino et al., SBRT was evaluated in the bridge to transplant setting as well as a definitive therapy in transplant ineligible patients. Sixty patients with HCC confined to the liver and CTP-A or CTP-B liver disease were treated to 44 Gy in 3 fractions or 40 Gy in 5 fractions, respectively. Most tumors were small (≤6 cm), with a median tumor diameter of 3.2 cm. At a median follow-up of 27 months, 2-year local control was reported as 90%, progression free survival was 48% and overall survival was 67%. Following SBRT, 23 patients underwent liver transplant (55).
A retrospective analysis by Facciuto et al., involved 27 patients with unresectable HCC totaling 39 lesions and CTP-A or -B cirrhosis who were treated with SBRT prior to OLT. Dose and fractionated ranged from 28 Gy in 4 fractions to 36 Gy in 2 fractions, with most patients receiving 28 Gy in 4 fractions. Seventeen patients with a total of 22 lesions underwent OLT. In addition to radiographic review of response to SBRT, response to treatment of lesions in patients who underwent OLT was also assessed pathologically. On radiographic review of 27 of the 39 treated lesions, 30% showed a CR, 7% showed a PR and 56% were stable. Only 7% showed progression of disease. On pathologic review of 22 of the treated lesions in the 17 transplanted patients, 37% showed either a complete or partial response; whereas, 63% showed no response (defined as less than 30% tumor necrosis) at a mean time of four months after SBRT (56).
Most recently, in sequential phase I (Trial 1) and II (Trial 2) trials at Princess Margaret Hospital by Bujold et al., SBRT was evaluated in the treatment of 102 patients with HCC and CTP-A liver disease. Trial 1 had no tumor number or size limits. In Trial 2, no more than five discrete liver tumors were allowed with a maximal dimension of 15 cm. Patients were treated to doses between 24 and 54 Gy in 6 fractions, with a median dose of 36 Gy. Local control at 1 year was reported at 87% with 11 patients achieving a CR, 44 patients with a partial response and 45 patients with stable disease. At a median follow-up of 31.4 months, 67 patients had died. Overall median survival was 17 months and on multivariate analysis, absence of tumor vascular thrombosis (TVT) and remaining on Trial 2 were associated with improved overall survival (57,58).
Liver metastases
In an interim analysis of a multi-institutional, phase I/II prospective trial, Kavanagh et al. reported excellent in-field local control using SBRT for liver metastases. Thirty-nine patients with tumors <6 cm in maximum diameter and a total of ≤3 lesions were included. Dose to 700 cm3 of the normal liver was limited to ≤15 Gy. Lesions were treated to 60 Gy in three 20 Gy-fractions over the course of 3-14 days. At a median follow-up of 18-months, local control for 28 of the lesions was 93% (10).
In a phase I/II study by Dawson et al. at Princess Margaret Hospital, dose-adjusted SBRT was used to treat 79 patients with either primary or metastatic hepatic malignancies. Forty-five patients with primary liver malignancies were treated including 33 patients with HCC and 12 patients with IHC. Thirty-four patients had liver metastases. Tumors ranged in size from 2.9 to 3,088 cc, with a median size of 293 cc. Prescription dose was adjusted based on a normal tissue complication probability (NTCP) model to limit estimated risk of RILD. Doses ranged between 24 and 57 Gy, with a median dose of 36.6 Gy. All patients were treated in 6 fractions. The primary objectives of the study were to determine the rate of RILD and severe toxicities and to stratify the risks based on both diagnosis and effective liver volume irradiated. The final results of this trial are not yet published. However, as of 2006, dose-limiting toxicity had not been observed. The conclusion at that time was based on initial analysis individualized, image-guided, iso-NTCP liver SBRT appears feasible (23).
Hoyer et al. looked at the use of SBRT in the treatment of metastases specifically from CRC primaries in a phase II, prospective study. Forty-four of the 64 patients had hepatic metastases. Lesions ranged in size from 10 to 88 mm, with a median size of 35 mm. All lesions, including extra-hepatic lesions, were treated to 45 Gy in 3 fractions. At a median follow-up of 4.3 years, 2-year tumor based actuarial local control was reported as 79%, but because several patients had more than one metastasis, patient based local control was lower at 64%. Two-year progression free survival was 19% with a median time to progression of 6.5 months. Overall survival was reported as 67%, 38%, 22%, 13%, and 13% at 1, 2, 3, 4 and 5 years following SBRT (59).
In a retrospective study by Katz et al., 69 patients with a total of 174 hepatic metastases were treated with SBRT. Twenty-eight patients received concurrent chemotherapy. Lesions ranged in size from 0.6 to 12.2 cm, with a median maximum tumor diameter of 2.7 cm. Doses ranged from 30 to 55 Gy, with a median dose of 48 Gy and a preferred fractionation of 50 Gy in 5 fractions. Dose was adjusted for preexisting, but non-malignant liver disease. At a median follow-up 14 months, 10- and 20-month local control was reported as 76% and 57%, respectively. Most patients (75%) developed additional lesions in the liver, with a median time to progression of 6.6 months. Progression-free survival was reported as 46% and 24% at 6 and 12 months, respectively. The median overall survival was 14.5 months (60).
Lee et al. conducted a phase I study of 68 patients with CTP-A liver disease and unresectable liver metastases of variable sizes using individualized SBRT doses that were adjusted for estimated risk of RILD. Tumors ranged in size from 1.19 to 3,090 cc, with a median volume of 75.2 cc. Lesions were treated to doses between 27.7 and 60 Gy, with a median dose of 41.8 Gy in 6 fractions. One-year local control was reported as 71%. Median overall survival was 17.6 months, however, the median survival of patients with CRC liver metastases was slightly shorter at 14.6 months (16).
In a multi-institutional, phase I/II trial by Rusthoven et al., excellent local control was reported using 60 Gy in 3 fractions for lesions ≤3 cm. Forty-seven patients with 63 metastatic liver lesions were included. Tumor size ranged from 0.4 to 5.8 cm, with a median maximum tumor diameter of 2.7 cm. In the first phase of the study, dose was escalated from 36 to 60 Gy in 3 fractions. The second phase of the study used 60 Gy in 3 fractions. At a median follow-up of 16 months, 1- and 2-year local control was reported as 95% and 92%, respectively. For lesions ≤3 cm, 2-year local control was 100%. Median overall survival was reported as 20.5 months. For favorable primaries, such as breast, CRC, renal, carcinoid, gastrointestinal stromal tumor, and sarcoma, however, median survival was longer at 32 months (18).
Recently, in a phase I, dose-escalation study by Goodman et al., single-fraction SBRT was evaluated in the treatment of unresectable, primary and metastatic hepatic malignancies. Twenty-six patients with CTP-A liver disease, ≤5 lesions and a maximum tumor diameter of ≤5 cm were included. Nineteen patients had hepatic metastases, including six metastases from CRC. Size ranged from 0.8-146.6 cc, with a median size of 32.6 cc. Lesions were treated to doses between 18 and 30 Gy in 4 Gy-intervals. At a median follow-up of 17 months, 1-year local control was approximately 77%. Two-year actuarial overall survival was 50.4% and median survival was 28.6 months (61).
In another phase I, dose-escalation trial by Rule et al., 27 patients with 37 small liver metastases, adequate hepatic function, and less than 5 lesions, were treated using SBRT. Tumors ranged in size from 0.4-7.8 cm, with a median diameter of 2.5 cm. Three cohorts of nine patients were treated to 30 Gy in 3 fractions, 50 Gy in 5 fractions or 60 Gy in 5 fractions. Dose to 700 cm3 of the normal liver was limited to <21 Gy. Two-year local control rates for the 30-, 50- and 60-Gy cohorts were reported as 56%, 89% and 100%, respectively. Median overall survival for all groups was 37 months and 2-year overall survival for the 30-, 50- and 60-Gy cohorts was 56%, 67% and 50%, respectively (62).
Toxicities
The most common complication of liver radiation is RILD, or radiation hepatitis. Originally described by Reed et al., RILD is a syndrome of fatigue, right upper quadrant pain, ascites, anicteric hepatomegaly and elevated transaminases (63). The syndrome typically occurs within 1-2 months of treatment and is associated with total liver irradiation at doses greater than 30-35 Gy in standard 2 Gy fractions. Early studies of normal tissue tolerances by Emami et al. found that whole liver radiation to 30 Gy in 2 Gy fractions was associated with a 5% risk of liver failure within 5 years; whereas, whole radiation to 40 Gy was associated with a 50% risk of RILD (64). Despite this risk of inducing more rapid liver failure with radiation, most of the early SBRT studies found this risk to be minimal with proper patient selection and strict dose-volume constraints (9,10,14,49,59,65).
In a recent meta-analysis by Sawrie et al., toxicity data was compiled from several of the earlier prospective trials involving SBRT for HCC and liver metastases (11). Toxicity was correlated with the dose-volume constraints, calculated BED and single-fraction equivalent doses (SFED) for the liver and surrounding organs at risk including the kidney, spinal cord, stomach, bowel, esophagus and heart. Most of the earlier studies limited the dose to 30-33% of the liver to between 7 and 21 Gy. With this constraint, the crude rate of RILD was found to be approximately 2.4%. Other liver related toxicities included portal hypertension, ascites, and elevated liver enzymes (49). Mendez Romero reported a single incidence of grade 5 liver toxicity in a patient with HCC, cirrhosis and hepatitis B virus infection. In these studies, the stomach was constrained to doses between 7 and 30 Gy. Grade 1 and 2 loss of appetite and nausea were relatively common, with toxicity being more severe for lesions located closer to the stomach. Diarrhea was a common bowel-related toxicity. One study reported duodenal ulceration and colonic perforation, however, these episodes occurred at bowel doses greater than 30 Gy (48). Reported skin toxicity included erythema, pain, dermatitis and one study reported skin breakdown six months post treatment (10). In addition to organ-related toxicities, constitutional toxicities such as fatigue, fever, chills and analgesia were common, but mild (9,65). Renal, cardiac, esophageal and spinal cord related toxicity was nominal in all studies.
Future directions
Currently, the role of SBRT in hepatic malignancies is primarily limited to settings in which resection is not feasible. No study has yet addressed SBRT in the setting of potentially resectable liver metastases. There is an ongoing multicenter randomized phase III trial (RAS study) of liver SBRT vs. RFA for patients with CRC liver metastases by the International Liver Tumor Group (www.livertumor.dk).
The role of SBRT in combination with small molecules with activity against HCC is currently under investigation in a large multi-center cooperative group randomized clinical trial (RTOG 1112). Sorafenib is a small molecule, tyrosine kinase inhibitor (TKI) which has been shown in two randomized trials [Sorafenib HCC Assessment Randomized Protocol (SHARP) (8) and the Asian Pacific Trial (66)], to improve survival in patients with advanced BCLC stage HCC. Sorafenib blocks angiogenesis through its potent activity against the c-raf, VEGFfr2/3 and PDGF-alpha kinases. The SHARP trial, which was comprised of 602 HCC patients, found an improvement in median survival from 7.9 to 10.7 months and median time to progression from 2.8 to 5.5 months in the sorafenib arm compared to placebo, with no difference in adverse events between the two treatment arms. In the Asian-Pacific trial, overall median survival improved from 4.2 to 6.5 months. In both of these trials, the majority of patients ultimately progressed in the liver and died of liver failure. The high-prevalence of progression in the liver provided the rationale for RTOG 1112 which adds local therapy (SBRT) to sorafenib. Despite this rationale, there are few retrospective or prospective studies on the combination of sorafenib or similar agents with RT. One retrospective review by Chi and colleagues in Taiwan of 23 patients with advanced HCC treated with RT to a median dose of 52.5 Gy in 15 fractions and sunitinib which is a TKI with a mechanism of action similar to sorafenib reported an objective response rate of 74%, a median survival of 16 months, and a 1-year survival rate of 70% (67).
Two additional phase I studies (one for patients with liver metastases and the other for those with HCC) combining 6 fractions SBRT plus dose-escalation of sorafenib have provided insight into how these treatments can be safely combined both in future clinical trials and in off-protocol clinical practice (68,69). In the HCC trial, 12 patients were evaluable for post-treatment toxicity after receiving continued sorafenib post-SBRT. There was no dose limiting toxicity (DLT) in the three evaluable HCC patients treated with SBRT with a low effective liver volume (Veff 30%) combined with 400 mg sorafenib. In patients with a liver Veff of 30-60%, 2 of 3 evaluable patients treated with sorafenib 400 mg daily developed DLT (grade 3 small bowel obstruction and grade 3 GI bleed); thus, sorafenib was de-escalated to 200 mg daily. In the liver metastases trial, there was no DLT among the 15 evaluable patients (3 at dose level 200 mg twice a day, 6 at dose level 600 mg and 6 at 800 mg for 4 weeks). In light of these data, sorafenib will be delivered following RT rather than concurrent with RT in RTOG 1112 to reduce the risk of toxicity (68,69).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Catherine T. Frenette) for the series “Liver Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.12.03). The series “Liver Cancer” was commissioned by the editorial office without any funding or sponsorship. JHG serves as an unpaid editorial board member of Translational Cancer Research. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- SEER Cancer Statistics 1975-2010. National Institutes of Health, 2013.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- American Cancer Society. eds. Cancer Facts & Figures 2013. Atlanta: American Cancer Society, 2013.
- Cabibbo G, Enea M, Attanasio M, et al. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology 2010;51:1274-83. [PubMed]
- Ruers T, Bleichrodt RP. Treatment of liver metastases, an update on the possibilities and results. Eur J Cancer 2002;38:1023-33. [PubMed]
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999;30:1434-40. [PubMed]
- Cárdenes HR. Role of stereotactic body radiotherapy in the management of primary hepatocellular carcinoma. Rationale, technique and results. Clin Transl Oncol 2009;11:276-83. [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [PubMed]
- Herfarth KK, Debus J, Lohr F, et al. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol 2001;19:164-70. [PubMed]
- Kavanagh BD, Schefter TE, Cardenes HR, et al. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol 2006;45:848-55. [PubMed]
- Sawrie SM, Fiveash JB, Caudell JJ. Stereotactic body radiation therapy for liver metastases and primary hepatocellular carcinoma: normal tissue tolerances and toxicity. Cancer Control 2010;17:111-9. [PubMed]
- Wulf J, Guckenberger M, Haedinger U, et al. Stereotactic radiotherapy of primary liver Cancer and hepatic metastases. Acta Oncol 2006;45:838-47. [PubMed]
- Cárdenes HR, Price TR, Perkins SM, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol 2010;12:218-25. [PubMed]
- Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2008;26:657-64. [PubMed]
- Dawood O, Mahadevan A, Goodman KA. Stereotactic body radiation therapy for liver metastases. Eur J Cancer 2009;45:2947-59. [PubMed]
- Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol 2009;27:1585-91. [PubMed]
- Russell AH, Clyde C, Wasserman TH, et al. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: results of the RTOG dose escalating protocol. Int J Radiat Oncol Biol Phys 1993;27:117-23. [PubMed]
- Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27:1572-8. [PubMed]
- Penna C, Nordlinger B. Colorectal metastasis (liver and lung). Surg Clin North Am 2002;82:1075-90. x-xi. [PubMed]
- Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 1989;62:679-94. [PubMed]
- Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 2002;53:810-21. [PubMed]
- Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys 1989;16:1623-30. [PubMed]
- Dawson LA, Eccles C, Craig T. Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncol 2006;45:856-64. [PubMed]
- Kitamura K, Shirato H, Seppenwoolde Y, et al. Tumor location, cirrhosis, and surgical history contribute to tumor movement in the liver, as measured during stereotactic irradiation using a real-time tumor-tracking radiotherapy system. Int J Radiat Oncol Biol Phys 2003;56:221-8. [PubMed]
- Case RB, Moseley DJ, Sonke JJ, et al. Interfraction and intrafraction changes in amplitude of breathing motion in stereotactic liver radiotherapy. Int J Radiat Oncol Biol Phys 2010;77:918-25. [PubMed]
- Park JC, Park SH, Kim JH, et al. Liver motion during cone beam computed tomography guided stereotactic body radiation therapy. Med Phys 2012;39:6431-42. [PubMed]
- Seppenwoolde Y, Wunderink W, Wunderink-van Veen SR, et al. Treatment precision of image-guided liver SBRT using implanted fiducial markers depends on marker-tumour distance. Phys Med Biol 2011;56:5445-68. [PubMed]
- Wunderink W, Méndez Romero A, Seppenwoolde Y, et al. Potentials and limitations of guiding liver stereotactic body radiation therapy set-up on liver-implanted fiducial markers. Int J Radiat Oncol Biol Phys 2010;77:1573-83. [PubMed]
- Méndez Romero A, Zinkstok RT, Wunderink W, et al. Stereotactic body radiation therapy for liver tumors: impact of daily setup corrections and day-to-day anatomic variations on dose in target and organs at risk. Int J Radiat Oncol Biol Phys 2009;75:1201-8. [PubMed]
- Park W, Lim DH, Paik SW, et al. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2005;61:1143-50. [PubMed]
- Lo SS, Dawson LA, Kim EY, et al. Stereotactic body radiation therapy for hepatocellular carcinoma. Discov Med 2010;9:404-10. [PubMed]
- Kirilova A, Lockwood G, Choi P, et al. Three-dimensional motion of liver tumors using cine-magnetic resonance imaging. Int J Radiat Oncol Biol Phys 2008;71:1189-95. [PubMed]
- Dawson LA, Eccles C, Bissonnette JP, et al. Accuracy of daily image guidance for hypofractionated liver radiotherapy with active breathing control. Int J Radiat Oncol Biol Phys 2005;62:1247-52. [PubMed]
- Hawkins MA, Brock KK, Eccles C, et al. Assessment of residual error in liver position using kV cone-beam computed tomography for liver cancer high-precision radiation therapy. Int J Radiat Oncol Biol Phys 2006;66:610-9. [PubMed]
- Yue J, Sun X, Cai J, et al. Lipiodol: a potential direct surrogate for cone-beam computed tomography image guidance in radiotherapy of liver tumor. Int J Radiat Oncol Biol Phys 2012;82:834-41. [PubMed]
- Eccles CL, Dawson LA, Moseley JL, et al. Interfraction liver shape variability and impact on GTV position during liver stereotactic radiotherapy using abdominal compression. Int J Radiat Oncol Biol Phys 2011;80:938-46. [PubMed]
- Heinzerling JH, Anderson JF, Papiez L, et al. Four-dimensional computed tomography scan analysis of tumor and organ motion at varying levels of abdominal compression during stereotactic treatment of lung and liver. Int J Radiat Oncol Biol Phys 2008;70:1571-8. [PubMed]
- Wunderink W, Méndez Romero A, de Kruijf W, et al. Reduction of respiratory liver tumor motion by abdominal compression in stereotactic body frame, analyzed by tracking fiducial markers implanted in liver. Int J Radiat Oncol Biol Phys 2008;71:907-15. [PubMed]
- Herfarth KK, Debus J, Lohr F, et al. Extracranial stereotactic radiation therapy: set-up accuracy of patients treated for liver metastases. Int J Radiat Oncol Biol Phys 2000;46:329-35. [PubMed]
- Wulf J, Hädinger U, Oppitz U, et al. Stereotactic radiotherapy of extracranial targets: CT-simulation and accuracy of treatment in the stereotactic body frame. Radiother Oncol 2000;57:225-36. [PubMed]
- Wulf J, Hädinger U, Oppitz U, et al. Impact of target reproducibility on tumor dose in stereotactic radiotherapy of targets in the lung and liver. Radiother Oncol 2003;66:141-50. [PubMed]
- Shirato H, Shimizu S, Kitamura K, et al. Four-dimensional treatment planning and fluoroscopic real-time tumor tracking radiotherapy for moving tumor. Int J Radiat Oncol Biol Phys 2000;48:435-42. [PubMed]
- Cho B, Poulsen PR, Sloutsky A, et al. First demonstration of combined kV/MV image-guided real-time dynamic multileaf-collimator target tracking. Int J Radiat Oncol Biol Phys 2009;74:859-67. [PubMed]
- Liu W, Wiersma RD, Mao W, et al. Real-time 3D internal marker tracking during arc radiotherapy by the use of combined MV-kV imaging. Phys Med Biol 2008;53:7197-213. [PubMed]
- Park JC, Park SH, Kim JH, et al. Four-dimensional cone-beam computed tomography and digital tomosynthesis reconstructions using respiratory signals extracted from transcutaneously inserted metal markers for liver SBRT. Med Phys 2011;38:1028-36. [PubMed]
- Wiersma RD, Mao W, Xing L. Combined kV and MV imaging for real-time tracking of implanted fiducial markers. Med Phys 2008;35:1191-8. [PubMed]
- Berber B, Ibarra R, Snyder L, et al. Multicentre results of stereotactic body radiotherapy for secondary liver tumours. HPB (Oxford) 2013. [Epub ahead of print].
- Blomgren H, Lax I, Näslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995;34:861-70. [PubMed]
- Méndez Romero A, Wunderink W, Hussain SM, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. Acta Oncol 2006;45:831-7. [PubMed]
- Choi BO, Jang HS, Kang KM, et al. Fractionated stereotactic radiotherapy in patients with primary hepatocellular carcinoma. Jpn J Clin Oncol 2006;36:154-8. [PubMed]
- Takeda A, Takahashi M, Kunieda E, et al. Hypofractionated stereotactic radiotherapy with and without transarterial chemoembolization for small hepatocellular carcinoma not eligible for other ablation therapies: Preliminary results for efficacy and toxicity. Hepatol Res 2008;38:60-9. [PubMed]
- Louis C, Dewas S, Mirabel X, et al. Stereotactic radiotherapy of hepatocellular carcinoma: preliminary results. Technol Cancer Res Treat 2010;9:479-87. [PubMed]
- Kwon JH, Bae SH, Kim JY, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer 2010;10:475. [PubMed]
- Seo YS, Kim MS, Yoo SY, et al. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J Surg Oncol 2010;102:209-14. [PubMed]
- Andolino DL, Johnson CS, Maluccio M, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2011;81:e447-53. [PubMed]
- Facciuto ME, Singh MK, Rochon C, et al. Stereotactic body radiation therapy in hepatocellular carcinoma and cirrhosis: evaluation of radiological and pathological response. J Surg Oncol 2012;105:692-8. [PubMed]
- Bujold A, Dawson LA. Stereotactic radiation therapy and selective internal radiation therapy for hepatocellular carcinoma. Cancer Radiother 2011;15:54-63. [PubMed]
- Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 2013;31:1631-9. [PubMed]
- Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol 2006;45:823-30. [PubMed]
- Katz AW, Carey-Sampson M, Muhs AG, et al. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys 2007;67:793-8. [PubMed]
- Goodman KA, Wiegner EA, Maturen KE, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys 2010;78:486-93. [PubMed]
- Rule W, Timmerman R, Tong L, et al. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol 2011;18:1081-7. [PubMed]
- Reed GB Jr, Cox AJ Jr. The human liver after radiation injury. A form of veno-occlusive disease. Am J Pathol 1966;48:597-611. [PubMed]
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109-22. [PubMed]
- Wulf J, Hädinger U, Oppitz U, et al. Stereotactic radiotherapy of targets in the lung and liver. Strahlenther Onkol 2001;177:645-55. [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [PubMed]
- Chi KH, Liao CS, Chang CC, et al. Angiogenic blockade and radiotherapy in hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2010;78:188-93. [PubMed]
- Dawson LA, Brade A, Cho C, et al. Phase I Study of Sorafenib and SBRT for advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2012;84:S10-S11.
- Brade A, Kim J, Brierley J, et al. Phase I Study of Sorafenib and Whole-liver Radiation Therapy (WLRT) or Stereotactic Body Radiation Therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys 2012;84:S11-S12.


