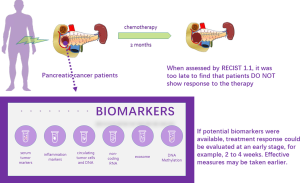
Potential biomarkers to evaluate therapeutic response in advanced pancreatic cancer
Background
Pancreatic adenocarcinoma is the fourth leading cause of cancer deaths in the United States and Europe (1). With its poor prognosis, pancreatic cancer is expected to become the second-leading cause of cancer-related deaths in the US by the year 2020 (2). Only 10–20% of patients are resectable when they are first diagnosed (3). Pancreatic cancer is a systematic rather than a localized disease. For most patients, chemotherapy is the fundamental treatment. Two significant regimen discoveries have led to modest improvement in survival, with the longest median overall survival time of 11.1 months reported in patients undergoing FOLFIRINOX regimens (4), and the second longest overall survival time of 8.5 months reported in patients undergoing regimens of gemcitabine with nab-paclitaxel (5). These represent clinically meaningful improvements over the prior standard of care, which was a regimen of gemcitabine alone. In addition, radiotherapy also resulted in markedly increased survival in locally advanced and metastatic pancreatic cancer (6).
Although surgery, chemotherapy, and radiotherapy are the main treatment approaches, overall survival remains less than a year. Novel therapies including immunotherapy and targeted therapy are appearing. A number of studies have focused on immunotherapy for pancreatic cancer, and regimens targeting programmed death 1 or programmed death 1 ligand 1 (PD-1/PD-L1), known as immune checkpoints, are emerging for the treatment of various cancers. However, single-agent checkpoint inhibitor treatment has poor outcomes in pancreatic cancer, and combining it with surgery, chemotherapy, radiotherapy or other immunotherapies could improve its efficacy (7). Other promising immunotherapies include the cancer vaccine known as GVAX (8) and chimeric antigen receptor T-cell therapy (CART) (9). The tumor microenvironment of pancreatic cancer is special because it is richer in stroma than other malignant tumors. Treatment regimens can be categorized into therapies enhancing drug delivery, anti-angiogenesis therapies, and metabolism-associated regimens. Therapies targeting signaling pathways are also under investigation (10-12).
Assessing therapeutic response is important in treating advanced pancreatic cancer. Radiographic assessment is considered as the standard of care for any patient on systemic therapy and is part of established clinical guidelines. The assessment of therapeutic response can assist with more accurate and individualized decisions, allowing patients to receive the maximum benefit and minimum damage. In other words, clinicians could make medical decisions based on therapeutic response to stop administering an ineffective medicine, to strengthen or continue an effective medicine, or to change to an available second-line regimen. Patient treatment can also be individualized, and invalid treatment may be avoided, leading to more alleviative treatment and life-support care. We lack knowledge about tumor biology, and the division of tumors into responsive or nonresponsive to treatment can help us understand them. A specific tumor gene expression profile or genotype (e.g., mutations or polymorphisms) may be correlated with tumor response to a particular therapy. The ideal predictive biomarker would be noninvasive, inexpensive, and effective at identifying high-risk non-responsive patients at an early stage.
Traditional serum biomarkers, especially CA19-9, play an important role in the management of patients with pancreatic cancer. Because of the development of modern technologies, novel biomarkers have been investigated and show promising potential. Non-coding RNA, consisting of long non-coding RNA (lncRNA) and microRNA (miRNA), has the unique potential to predict treatment response. Circulating tumor cells (CTC) and DNA detected by tumor biopsy may also potentially serve as biomarkers.
In this review, we first discuss the gold standard for assessing tumor response to chemotherapy, RECIST 1.1. Second, a variety of promising biomarkers employed in evaluating tumor response to treatment are listed by category. We divided these potential biomarkers into 7 groups: serum tumor biomarkers, inflammation markers, CTCs and DNA, non-coding RNA, exosomes, DNA methylation, and other markers (Figure 1).
Current method of evaluating treatment response
Various imaging techniques are applied to screen, diagnose, and stage pancreatic cancer, as well as to evaluate the response to therapy. In pancreatic cancer, radiologic assessment of tumor response is still the fundamental and basic method, and measuring the amount of tumor shrinkage using CT represents the current gold standard for the evaluation of the effectiveness of chemotherapy. However, limitations remain in these methods. Images may be confused by desmoplastic reactions within or around the tumor; thus, the degree of response may be underestimated. Currently, RECIST guidelines (version 1.1) are the standard criteria for evaluating tumor response to therapy.
High rates of false-positives and escalating costs are the major shortcomings of imaging in assessing response to therapy. Thus, unless a more sensitive and specific procedure is found, these methods are not valuable enough to use in the assessment of the response to therapy at an early stage.
Biomarkers
Serum tumor markers
CA 19-9
Efforts to develop early response tests are focused on serum biomarkers. Among a variety of laboratory tests, CA 19-9 is the optimal biomarker for evaluating early response and thus is in widespread clinical use. Since the 1980s, serum CA19-9 has been widely used as a marker of pancreatic cancer (13). However, a small proportion of patients cannot produce this carbohydrate tumor-associated antigen, and thus, their levels of serum CA 19-9 are normal, and this method is not appropriate for them (14). This is because they belong some of the genetically specific population whose Lewis antigens, antigens related to CA 19-9, are negative. In most patients, this test is effective.
Assessing the response to treatment has been the primary accepted use for CA19-9. Studies have shown that baseline or changed values of CA19-9 could serve as biomarkers in evaluating therapeutic responses. Hammad et al. reported that baseline levels of CA19-9 could predict survival for pancreatic cancer patients; however, changed values of CA19-9 one month after therapy did not predict treatment response (15). Meanwhile, a study claimed that a decrease in the level of CA19-9 two months after therapy could predict treatment response (16). Robert et al. also confirmed that a decrease in the level of CA19-9 eight weeks after treatment presented better OS, PFS, and objective response rate (ORR) both in the gemcitabine group and the FOLFIRINOX group, especially for a sharp decrease ≥20% (17). That predicted the response to treatment a month earlier than it was possible to do with the RECIST 1.1 criteria. Some studies analyzed the use of CA19-9 from a different angle. Tsutsumi et al. proposed that altered levels of CA19-9 >25% for a month or >10% for two continuous months predicted treatment response (18). The baseline level of CA19-9 is considered an independent prognostic factor for survival, and a decrease in the level of CA19-9 is significantly related to survival during chemotherapy, especially with advanced stages of cancer. However, several studies have suggested that neither early decreases in the level of CA19-9 nor the lowest level of CA19-9 is related to prolonged overall survival, whereas in the gemcitabine contained arms in contrast to previously smaller studies; therefore, CA19-9 is not a novel end-point in clinical use (19,20). Several prospective studies have provided evidence that CA19-9 is a useful biomarker for the response to the most commonly used systematic regimens, so it may provide useful information in predicting treatment response.
CA125
CA125 was the first and is the most frequently used biomarker for ovarian cancer detection, and its clinical use in pancreatic cancer has been discovered in recent years. Liu et al. reported that CA125 was superior to CA19-9 in predicting resectability and was a novel predictor for metastasis in patients with pancreatic cancer (21). High levels of CA125 can indicate unresectable cancer and metastasis in pancreatic cancer, even in those who have already been evaluated by CT. A decrease in the level of CA125 may indicate a decrease in the tumor burden, and it could be a potential novel treatment response marker.
The value of CA125 in the monitoring of therapeutic response has been identified. A study reported that changes in CA125 levels correlated with treatment effects and disease progression (22). Similar to CA19-9, dynamic changes in the levels of CA125 can act as a biomarker to monitor the response of therapy. Measuring the serum CA125 level could potentially predict the therapeutic response, and its potential in this area needs further investigation.
Other tumor markers
CEA, CA72-4, and CA242 are also regularly measured in laboratory testing. Unlike CA19-9 and CA125, they are usually evaluated together, rather than individually. Combining serum tumor markers improves predictive accuracy and ability. A recent study has shown that a combination of serum tumor biomarkers consisting of CA19-9, CA125, and CEA predicts poor outcomes for surgical candidates (23). These patients are classified as having tri-positive pancreatic cancer, and unfortunately do not respond to the current therapeutic methods and have poor outcomes. Some clinicians have proposed using a combination of CEA and CA19-9 (24), while some have suggested a combination of CA199, CA125, CEA, and CA72-4. It is difficult to determine which combination is the best solution, but the evidence indicates that a combination of markers is more accurate than a single marker.
Inflammation markers
In 1863, the presence of leukocytes was found in tumor tissues for the first time by Rudolf Virchow, and possible connections between inflammation and cancer were discovered (25). However, only during the last decade has there been enough evidence to support the vital role of inflammation in tumorigenesis, and some mechanisms responsible for the association have been preliminarily discovered. Both tumor cells and the related host cells could generate inflammatory cytokines and chemokines, which participate in disease progression (26). Recently, inflammation has been recognized as a hallmark of cancer (27). For example, interleukin-1 and interleukin-6, two typical proinflammatory mediators, are up-regulated in pancreatic cancer patients (28).
Several prognostic factors based on local or systemic inflammation related to cancer have been explored, including the neutrophil-to-lymphocyte ratio (NLR), C-reactive protein (CRP) and its combination with albumin, the modified Glasgow prognostic score (mGPS), the prognostic nutritional index (PNI), the platelet-to-lymphocyte ratio (PLR), and the prognostic index (PI). Changes in systematic inflammation or the inflammatory response to tumor-development are easy to measure.
NLR
High NLRs are associated with poor outcomes in several solid tumors (29,30), including breast cancer, renal cell carcinoma, colorectal cancer, melanoma, and gastric carcinoma. The impacts of the baseline NLR and changes in the NLR in patients undergoing therapy that could predict treatment response have captured researchers’ attention, and promising results have recently been achieved, although the mechanisms remain unclear. One possible explanation is that increased numbers of neutrophils are thought to play a role in the inflammatory response and may inhibit the cytolytic activity of immune cells such as lymphocytes, activated T cells and natural killer cells (NKT) activity inhibition (31). Further investigation of these impacts is needed.
Former studies have shown that an increased baseline NLR independently predicts poor prognosis and shorter overall survival in patients with pancreatic cancer, regardless of whether it is operable or inoperable (32) and whether it is before chemotherapy or after chemotherapy (33). The clinical use of the baseline NLR in predicting treatment response will also be investigated in the future. However, the standard medical reference range varies from study to study. Luo selected an NLR of >3.1 as the cut-off value (33), while Goldstein and Tsai chose an NLR of >5 as the cut-off value (34,35). Other cut-off values, such as NLR >2, have been proposed by Ben (36). The use of different standards to judge whether NLR is high or low still occurs at present, and consensus on the issue is urgently needed.
CRP and mGPS
Kishi et al. suggested that CRP significantly predicted disease control in patients undergoing chemotherapy (37). So far, few studies have concentrated on its role in predicting treatment response. However, it is possible to deduce this role from another perspective, such as its usefulness in predicting prognosis. Mitsunaga et al. found that CRP was an independent predictor of survival in multivariate analyses in patients with metastatic pancreatic cancer (280 patients in the retrospective cohort, and 141 patients in the prospective cohort) (38).
Combining hypoalbuminemia and elevated levels of CRP, also called mGPS, has a better prognostic value in patients with advanced cancer. Typically, the presence of a systemic inflammatory response is generally measured by mGPS. The mGPS is calculated using albumin and CRP as follows: patients with both low albumin levels (<35 g/L) and elevated CRP levels (≥10 mg/L) are allocated a score of 2, patients with only elevated levels of CRP (≥10 mg/L) are allocated a score of 1, and those with normal CRP levels are allocated a score of 0 (39). Studies have reported predictive value of the mGPS scores for patients treated with surgery (40), chemoradiotherapy (41), and adjuvant chemotherapy (42). Some studies have combined mGPS scores and CA19-9 levels to predict treatment outcome and survival time (43). However, few studies have concentrated on patients with advanced pancreatic cancer who are receiving chemotherapy. One possible explanation is that chemotherapy itself may induce an inflammatory response, which can be measured by mGPS.
PNI
Nutritional status is a matter of great concern for both surgical and non-surgical patients. In 1981, Smale et al. first proposed the concept of PNI (44). Among patients with advanced pancreatic cancer, Geng et al. reported that low values of PNI indicate a shorter median overall survival (163 vs. 301 d, P<0.01) (45). This association is readily understandable, because poor nutritional status is correlated with poor prognosis. Increased PNI indicates a better nutritional status when undergoing chemotherapy; however, whether it could serve as a crucial early marker of the response to treatment needs further study.
PLR
Platelets are one of the most abundant cell types in peripheral blood. A new concept of “tumor-educated platelets” has been mentioned (46), which refers to the interaction between tumor cells and platelets through tumor-associated molecules. Several reports have suggested that preoperative PLR is an independent predictor of disease-free survival and overall survival in resectable patients with pancreatic ductal adenocarcinoma, and Yoshihiro proposed that a preoperative PLR >150 was an independent predictor of disease-free survival and overall survival after surgery (47). Meanwhile, Spolverato has suggested that low PLR values are associated with shorter OS in PDAC patients (48). However, some researchers hold the opposite opinion. Bhatti has suggested that PLR, unlike NLR, does not represent an independent predictor in PDAC patients (49). It is clear that the role of PLR is still controversial. In addition, most studies have focused on patients who are suitable for surgery, but the predictive values in those with advanced stages of cancer who are mainly treated with chemotherapy have been neglected.
Combination of inflammation markers
A single marker usually does not guarantee adequate sensitivity and specificity, and combinations of inflammation markers can maximize predictive values. Miura et al. created a new preoperative prognostic scoring (PPS) system (50), including preoperative platelet counts, preoperative CRP levels, and preoperative CA19-9 levels to predict the prognosis in patients receiving surgery with locally advanced pancreatic cancer. The criteria for the PPS system are shown in Table 1.
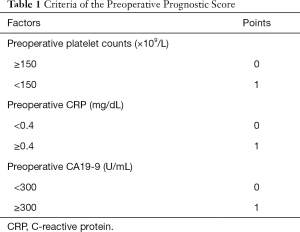
Full table
Qi et al. developed a scoring system based on peripheral lymphocytes, neutrophils and monocytes to evaluate the prognosis in advanced pancreatic cancer patients receiving chemotherapy (51). The definition of SIRI is: SIRI = N×M/L, where N, M, and L are the baseline values of peripheral neutrophil, monocyte, and lymphocyte counts, respectively.
Circulating tumor DNA (ctDNA) and CTCs
ctDNA
Pilot studies have also reported that ctDNA can be employed in monitoring treatment efficacy and predicting disease progression in the management of patients with advanced pancreatic cancer (52). Cheng et al. concluded that the identification of ctDNA indicated an early treatment response in advanced pancreatic cancer patients, suggesting that ctDNA could serve as a marker to monitor tumor response. Detectable ctDNA can be found in the plasma, which is a non-invasive method. Hadano et al. detected rare mutant tumor-derived KRAS genes in ctDNA and suggested that the presence of ctDNA predicts poor survival in pancreatic cancer patients (53). A study involving a large patient sample is needed to prove these findings.
CTC
Previous studies have reported that CTC can predict treatment response in the pharmacogenomic modeling of tumor tissue (54). Identification of these cells mainly depends on two methods; one uses the molecular analysis of blood, while the other uses specific antibodies to bind cell surface epithelial markers. Recently, several different subtypes of pancreatic cancer have been discovered, which may account for their heterogeneity in terms of therapeutic response (55,56). Positive CTC patients presented shorter overall survival and poor tumor differentiation in contrast to negative CTC patients with locally advanced pancreatic carcinoma (RR=2.5, P=0.01) (57). Another trial reported that the presence of CTC in blood from the portal vein could predict liver metastasis (58). However, few studies have been conducted to identify whether the baseline level of CTC or decrease in CTC suggest early treatment response.
Non-coding RNA
New technology involving the detection of non-coding RNA (59) will enable potential candidate response biomarkers to be used once the new methods are well established. Additionally, the development of high-throughput sequencing techniques has helped to reveal unannotated tumor-associated non-coding RNAs as specific and novel biomarkers of cancer.
miRNA
miRNAs are small noncoding regulatory RNAs that participate in several stages of human cancers and that are emerging as important modulators of responses to treatment in certain cancers, such as colorectal cancer, liver cancer, and ovarian cancer. The role of miRNAs in gemcitabine drug resistance has been examined (60). Several subtypes of miRNAs have been highlighted by researchers. Preis et al. also reported that miR-10b predicts the response to neoadjuvant therapy and outcomes in pancreatic cancer (61). Donahue et al. proposed that miR-21 could predict the response to treatment in PDAC patients who received 5-fluorouracil, but not those who received gemcitabine (62). Nevertheless, there is still much to be discovered regarding the clinical use of miRNAs. Other potentially useful miRNAs are miR-181c (63), miR-320c (64), and miR-101-3p (60). However, different miRNAs have different sensitivities and specificities. Most of these miRNAs have high sensitivities, making them suitable for use in evaluating treatment response, and miR-21 has a specificity of 85%, which has more power to assess responses to treatment.
lncRNA
There are fewer studies focusing on lncRNAs than on miRNAs. HOTAIR and MALAT1, two serum lncRNAs, have also been reported as being diagnostic and prognostic biomarkers. HOTAIR interacts with polycomb repressive complex 2 (PRC2), and several studies have reported that the overexpression of HOTAIR predicts poor survival in breast cancer, liver cancer, and colorectal cancer. Kim et al. compared levels of HOTAIR in pancreatic cancer tissues and non-tumor tissues, and showed that the knockdown of HOTAIR by gene interference deceased cell proliferation (65). Pang et al. also reported that the overexpression of MALAT1 serves as a predictive biomarker in pancreatic cancer patients (66). However, the value of miRNAs in predicting treatment response requires further investigation.
Exosomes
An exosomes is a type of extracellular vesicle, consisting of RNA and proteins, which is secreted into the extracellular space. Compared with normal cells, pancreatic cancer cells release more exosomes, which contain the two subtypes of molecules mentioned above. Pioneering research has shown that differential loading of exosomes, detected by quantitively analyzing the exosomal proteome, indicates different responses to therapy (67). An exosome called GPC1+ contains identical KRAS mutations, which are a common feature in pancreatic cancer.
Que et al. detected four miRNAs (miRNA-21, miRNA-155, miRNA-17-5p, and miRNA-196a) related to pancreatic cancer and found that the levels of miRNA-21 and the serum exosomal miRNA-17-5p were significantly higher in patients with pancreatic cancer, especially the latter (68). Madhavan et al. also reported that four miRNAs (miRNA-1246, miRNA-3976, miRNA-4306 and miRNA-4644) were significantly elevated in the serum exosomes of pancreatic cancer patients’ (69). Richards et al. reported that cancer-associated fibroblasts exposed to gemcitabine increased the release of exosomes, which was a chemo-resistance-inducing factor (70). Collectively, these findings show the potential for exosomes to be used in evaluating the therapeutic response of advanced pancreatic cancer.
DNA methylation
Methylation of DNA at cytosine-phosphate-guanine (CpG) island sites provides marker candidates that are more effective than individual DNA mutations. Next-generation sequencing has helped us to understand the epigenetic regulation of gene expression. Kisiel et al. proposed that methylated CD1D was a sensitive and specific marker for pancreatic cancer and claimed that the marker performance was superior to the identification of mutant KRAS, which was not sufficiently specific (71). Another study conducted by Yi et al. showed a high frequency of methylation in pancreatic cancers. Two genes, BNC1 and ADAMTS1, were identified by the application of a novel technique using a nanoparticle (72). Nones et al. detect another two genes, MET and ITGA2, and found that high levels of expression of these genes accompanied by hypomethylation predicted shorter survival times (73). From that perspective, aberrant DNA methylation and altered gene expression were found to play a vital role in pancreatic cancer tumorigenesis and provided potential biomarkers for therapeutic assessment by measuring the alteration of DNA methylation.
Other markers
Falco et al. reported that the intracellular anti-apoptotic protein BAG3 was only detected in tumor tissues, but in normal tissue or pericarcinomatous tissue (74). Detection of BAG3 could also serve as a potential marker to evaluate treatment response. In addition to identifying markers in serum samples, some studies have also selected urine or saliva as lab samples. Radon et al. established a novel, three-protein biomarker panel to detect early pancreatic cancer patients that consisted of LYVE-1, REG1A, and TFF1 (75). Signaling pathways have also provided potential markers. Zhong et al. found that the inhibition of p38 increased cancer cell proliferation and that functional p38 improved survival through the Jun amino-terminal kinases (JNK) pathway, which provided a rationale for the use of JNK inhibition in pancreatic cancer management (76). Methylation biomarker panels also have advantages. Yi et al. reported that promoter DNA methylation of BNC1 and ADAMTS1 is a potential biomarker for the detection of pancreatic cancer (72), although its use in assessing treatment response has not been clearly studied.
Discussion
Surgery, chemotherapy and radiotherapy remain the fundamental therapies for pancreatic cancer. Despite aggressive treatment regimens, only a modest improvement in survival has been achieved. Novel therapies including immunotherapy and targeted therapy are still under investigation. Radiographic assessment of tumor response is still considered the standard of care for any patient on systemic therapy and is part of established clinical guidelines. Currently, intensive efforts have been made to find an ideal alternative biomarker to evaluate tumor response during therapy. In this review, we discussed biomarkers that are potentially applicable to the evaluation of treatment response.
Currently, several studies have focused on this question. Patients identified as high-risk may accept CT or MRI evaluation earlier; thus, regimens not producing a response could be replaced by more effective treatments. In addition, potential toxicity and escalating costs could be avoided as well. The search for markers to predict the response of pancreatic cancer to treatment is an active area of urgent research.
The ideal model for predicting therapeutic response consists of a cluster of biomarkers of different types, such as serum biomarkers, inflammatory markers, and nucleic acids. A powerful cluster of biomarkers could not only be used to predict therapeutic response but also in each aspect of monitoring the status of patients. Moreover, a potential novel biomarker could even act as a therapeutic target. To achieve the goal of identifying more potential biomarkers, we should understand more about the mechanisms involving these molecules. Novel markers may not only merely act as a marker, but may also participate in the development of disease. These molecules could be detected in the nucleus, on the cell surface, in the blood, and so on. Therefore, more detailed knowledge about the cellular and molecular mechanisms involved is required. We need more support from basic medical science.
Whether a marker used to predicting the response to a treatment depends on when and how it changes during the treatment. If a change occurs four months after the initial chemotherapy, there is no benefit to using the marker. The changed levels must be observable fewer than three months after the initial treatment. In addition, different levels of change could indicate different levels of response. For instance, a decrease of over 20% in the level of CA19-9 predicts better PFS in pancreatic cancer (17). Further investigation is needed to identify novel markers.
Strong evidence for the prediction of treatment response has so far only been found for CA19-9, ctDNA, miRNA and exosomes. CA19-9, a traditionally used serum biomarker in pancreatic cancer, has shown particularly encouraging results. Prospective studies have proved that CA19-9 is a useful biomarker of tumor responses to the most commonly used systematic regimens, so it could provide useful information in predicting treatment responses. Other popular markers used for pancreatic cancer, such as CA125 and NLR, have not been studied with regards to their ability to predict responses to therapy. Novel biomarker should be identified to increase accuracy.
Apart from famous biomarker such as CA19-9, modern techniques have accelerated discovery of novel biomarkers, such as ctDNA, CTC, miRNAs, lncRNAs, exosomes, or epigenetic modifications. Most of these markers are non-invasive and have shown modest performance in predicting the prognosis of pancreatic cancer. Imaging surveillance remains the basic care of monitoring the response to treatment, and imaging markers could be applicable. Novel techniques such as CT texture analysis (CTTA), dual energy CT (DECT), and diffusion-weighted imaging (DWI) have appeared in some studies with large cohorts (77). PET-CT could monitor tumor changes in patients undergoing chemotherapy and radiotherapy (78). An advantage of PET-CT is that the metabolic changes can be detected before the tumor size shrinks. A recent study reported that the metabolic changes detected by PET-CT predicted better outcomes in pancreatic cancer (79). However, evidence is limited, and these techniques still require further study.
The final aim of evaluating the therapeutic response of tumors is to increase the overall survival of patients. With several studies conducted in breast and prostate cancer, the prediction program may have some influence on overall survival. Strategies for the management of patients are complex, and while the aim of predicting therapeutic responses is important, there are still many obstacles, such as the difficulty of determining a robust biomarker for false positives. Integrated scores may be a better choice; however, there is no widely accepted standard to meet this requirement.
In 5 years, we will have an ideal prediction model consisting of multi-dimensional markers. At that time, a new standard of chemotherapy may have been developed. However, the concept of personalized treatment should never be changed. Progress in molecular and cellular research will be of assistance in finding an appropriate resolution to this problem. In addition, multicenter clinical research studies can be undertaken to investigate our hypothesis.
Conclusions
Most patients with pancreatic cancer are not diagnosed until the disease has developed to an advanced stage. The current criteria for evaluating tumor response requires at least two months after the initial treatment, and by that time, some patients have progressed to an untreatable stage. We attempted to identify a marker that could be used to evaluate treatment response earlier than two months. A cluster of biomarkers, consisting of traditional serum biomarkers and other novel markers, was discussed. Different permutations and combinations of these markers could serve as useful modalities.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China grants [81370065, 81372653]; and a basic research project of the Science and Technology Commission of Shanghai Municipality (15JC1401200); and The National Science Fund for Distinguished Young Scholars [81625016].
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.01.24). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267 [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Yang SH, Guo JC, Yeh KH, et al. Association of radiotherapy with favorable prognosis in daily clinical practice for treatment of locally advanced and metastatic pancreatic cancer. J Gastroenterol Hepatol 2016;31:2004-12. [Crossref] [PubMed]
- Feng M, Xiong G, Cao Z, et al. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett 2017;407:57-65. [Crossref] [PubMed]
- Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 2015;33:1325-33. [Crossref] [PubMed]
- DeSelm CJ, Tano ZE, Varghese AM, et al. CAR T-cell therapy for pancreatic cancer. J Surg Oncol 2017;116:63-74. [Crossref] [PubMed]
- De Jesus-Acosta A, Laheru D, Maitra A, et al. A phase II study of the gamma secretase inhibitor RO4929097 in patients with previously treated metastatic pancreatic adenocarcinoma. Invest New Drugs 2014;32:739-45. [Crossref] [PubMed]
- Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene 2000;19:5662-79. [Crossref] [PubMed]
- Campbell PM, Groehler AL, Lee KM, et al. K-Ras promotes growth transformation and invasion of immortalized human pancreatic cells by Raf and phosphatidylinositol 3-kinase signaling. Cancer Res 2007;67:2098-106. [Crossref] [PubMed]
- Glenn J, Steinberg WM, Kurtzman SH, et al. Evaluation of the utility of a radioimmunoassay for serum CA 19-9 levels in patients before and after treatment of carcinoma of the pancreas. J Clin Oncol 1988;6:462-8. [Crossref] [PubMed]
- Luo G, Guo M, Jin K, et al. Optimize CA19-9 in detecting pancreatic cancer by Lewis and Secretor genotyping. Pancreatology 2016;16:1057-62. [Crossref] [PubMed]
- Hammad N, Heilbrun LK, Philip PA, et al. CA19-9 as a predictor of tumor response and survival in patients with advanced pancreatic cancer treated with gemcitabine based chemotherapy. Asia Pac J Clin Oncol 2010;6:98-105. [Crossref] [PubMed]
- Chiorean EG, Von Hoff DD, Reni M, et al. CA19-9 decrease at 8 weeks as a predictor of overall survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Ann Oncol 2016;27:654-60. [Crossref] [PubMed]
- Robert M, Jarlier M, Gourgou S, et al. Retrospective Analysis of CA19-9 Decrease in Patients with Metastatic Pancreatic Carcinoma Treated with FOLFIRINOX or Gemcitabine in a Randomized Phase III Study (ACCORD11/PRODIGE4). Oncology 2017;93: [PubMed]
- Tsutsumi K, Kawamoto H, Hirao K, et al. Monitoring of CA19-9 and SPan-1 can facilitate the earlier confirmation of progressing pancreatic cancer during chemotherapy. Pancreatology 2012;12:409-16. [Crossref] [PubMed]
- Hess V, Glimelius B, Grawe P, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol 2008;9:132-8. [Crossref] [PubMed]
- Bauer TM, El-Rayes BF, Li X, et al. Carbohydrate antigen 19-9 is a prognostic and predictive biomarker in patients with advanced pancreatic cancer who receive gemcitabine-containing chemotherapy: a pooled analysis of 6 prospective trials. Cancer 2013;119:285-92. [Crossref] [PubMed]
- Liu L, Xu HX, Wang WQ, et al. Serum CA125 is a novel predictive marker for pancreatic cancer metastasis and correlates with the metastasis-associated burden. Oncotarget 2016;7:5943-56. [PubMed]
- Myriokefalitaki E, Vorgias G, Vlahos G, et al. Prognostic value of preoperative Ca125 and Tag72 serum levels and their correlation to disease relapse and survival in endometrial cancer. Arch Gynecol Obstet 2015;292:647-54. [Crossref] [PubMed]
- Liu L, Xu H, Wang W, et al. A preoperative serum signature of CEA+/CA125+/CA19-9 ≥ 1000 U/mL indicates poor outcome to pancreatectomy for pancreatic cancer. Int J Cancer 2015;136:2216-27. [Crossref] [PubMed]
- Reitz D, Gerger A, Seidel J, et al. Combination of tumour markers CEA and CA19-9 improves the prognostic prediction in patients with pancreatic cancer. J Clin Pathol 2015;68:427-33. [Crossref] [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [Crossref] [PubMed]
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer 2004;4:540-50. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Ebrahimi B, Tucker SL, Li D, et al. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer 2004;101:2727-36. [Crossref] [PubMed]
- Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124 [Crossref] [PubMed]
- Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol 2013;23:200-7. [Crossref] [PubMed]
- Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol 1985;134:230-4. [PubMed]
- Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 2013;109:416-21. [Crossref] [PubMed]
- Luo G, Guo M, Liu Z, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol 2015;22:670-6. [Crossref] [PubMed]
- Tsai PL, Su WJ, Leung WH, et al. Neutrophil-lymphocyte ratio and CEA level as prognostic and predictive factors in colorectal cancer: A systematic review and meta-analysis. J Cancer Res Ther 2016;12:582-9. [Crossref] [PubMed]
- Goldstein D, El-Maraghi RH, Hammel P, et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst 2015;107:dju413 [Crossref] [PubMed]
- Ben Q, An W, Wang L, et al. Validation of the pretreatment neutrophil-lymphocyte ratio as a predictor of overall survival in a cohort of patients with pancreatic ductal adenocarcinoma. Pancreas 2015;44:471-7. [PubMed]
- Kishi T, Nakamura A, Itasaka S, et al. Pretreatment C-reactive protein level predicts outcome and patterns of failure after chemoradiotherapy for locally advanced pancreatic cancer. Pancreatology 2015;15:694-700. [Crossref] [PubMed]
- Mitsunaga S, Ikeda M, Shimizu S, et al. C-Reactive Protein Level Is an Indicator of the Aggressiveness of Advanced Pancreatic Cancer. Pancreas 2016;45:110-6. [Crossref] [PubMed]
- Proctor MJ, Morrison DS, Talwar D, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer 2011;104:726-34. [Crossref] [PubMed]
- Yamada S, Fujii T, Yabusaki N, et al. Clinical Implication of Inflammation-Based Prognostic Score in Pancreatic Cancer: Glasgow Prognostic Score Is the Most Reliable Parameter. Medicine (Baltimore) 2016;95:e3582 [Crossref] [PubMed]
- Kurahara H, Maemura K, Mataki Y, et al. Prognostication by inflammation-based score in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Pancreatology 2015;15:688-93. [Crossref] [PubMed]
- Morinaga S, Murakawa M, Katayama Y, et al. Glasgow Prognostic Score Predicts Clinical Outcomes in Patients with Pancreatic Cancer Undergoing Adjuvant Gemcitabine Monotherapy After Curative Surgery. Anticancer Res 2015;35:4865-70. [PubMed]
- Numata K, Morinaga S, Katayama Y, et al. Combining the Glasgow Prognostic Score and Serum Carbohydrate Antigen 19-9 Level Improves the Ability to Predict Early Recurrence in Resected Pancreatic Cancer Patients Receiving Adjuvant Gemcitabine. Anticancer Res 2016;36:2467-74. [PubMed]
- Smale BF, Mullen JL, Buzby GP, et al. The efficacy of nutritional assessment and support in cancer surgery. Cancer 1981;47:2375-81. [Crossref] [PubMed]
- Geng Y, Qi Q, Sun M, et al. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur J Surg Oncol 2015;41:1508-14. [Crossref] [PubMed]
- Best MG, Sol N, Kooi I, et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell 2015;28:666-76. [Crossref] [PubMed]
- Shirai Y, Shiba H, Sakamoto T, et al. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery 2015;158:360-5. [Crossref] [PubMed]
- Spolverato G, Maqsood H, Kim Y, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after resection for hepato-pancreatico-biliary malignancies. J Surg Oncol 2015;111:868-74. [Crossref] [PubMed]
- Bhatti I, Peacock O, Lloyd G, et al. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg 2010;200:197-203. [Crossref] [PubMed]
- Miura T, Hirano S, Nakamura T, et al. A new preoperative prognostic scoring system to predict prognosis in patients with locally advanced pancreatic body cancer who undergo distal pancreatectomy with en bloc celiac axis resection: a retrospective cohort study. Surgery 2014;155:457-67. [Crossref] [PubMed]
- Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 2016;122:2158-67. [Crossref] [PubMed]
- Tjensvoll K, Lapin M, Buhl T, et al. Clinical relevance of circulating KRAS mutated DNA in plasma from patients with advanced pancreatic cancer. Mol Oncol 2016;10:635-43. [Crossref] [PubMed]
- Hadano N, Murakami Y, Uemura K, et al. Prognostic value of circulating tumour DNA in patients undergoing curative resection for pancreatic cancer. Br J Cancer 2016;115:59-65. [Crossref] [PubMed]
- Yu M, Bardia A, Aceto N, et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 2014;345:216-20. [Crossref] [PubMed]
- Yu KH, Ricigliano M, Hidalgo M, et al. Pharmacogenomic modeling of circulating tumor and invasive cells for prediction of chemotherapy response and resistance in pancreatic cancer. Clin Cancer Res 2014;20:5281-9. [Crossref] [PubMed]
- Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518:495-501. [Crossref] [PubMed]
- Bidard FC, Huguet F, Louvet C, et al. Circulating tumor cells in locally advanced pancreatic adenocarcinoma: the ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol 2013;24:2057-61. [Crossref] [PubMed]
- Bissolati M, Sandri MT, Burtulo G, et al. Portal vein-circulating tumor cells predict liver metastases in patients with resectable pancreatic cancer. Tumour Biol 2015;36:991-6. [Crossref] [PubMed]
- Müller S, Raulefs S, Bruns P, et al. Next-generation sequencing reveals novel differentially regulated mRNAs, lncRNAs, miRNAs, sdRNAs and a piRNA in pancreatic cancer. Mol Cancer 2015;14:94. [Crossref] [PubMed]
- Fan P, Liu L, Yin Y, et al. MicroRNA-101-3p reverses gemcitabine resistance by inhibition of ribonucleotide reductase M1 in pancreatic cancer. Cancer Lett 2016;373:130-7. [Crossref] [PubMed]
- Preis M, Gardner TB, Gordon SR, et al. MicroRNA-10b expression correlates with response to neoadjuvant therapy and survival in pancreatic ductal adenocarcinoma. Clin Cancer Res 2011;17:5812-21. [Crossref] [PubMed]
- Donahue TR, Nguyen AH, Moughan J, et al. Stromal microRNA-21 levels predict response to 5-fluorouracil in patients with pancreatic cancer. J Surg Oncol 2014;110:952-9. [Crossref] [PubMed]
- Chen M, Wang M, Xu S, et al. Upregulation of miR-181c contributes to chemoresistance in pancreatic cancer by inactivating the Hippo signaling pathway. Oncotarget 2015;6:44466-79. [Crossref] [PubMed]
- Iwagami Y, Eguchi H, Nagano H, et al. miR-320c regulates gemcitabine-resistance in pancreatic cancer via SMARCC1. Br J Cancer 2013;109:502-11. [Crossref] [PubMed]
- Kim K, Jutooru I, Chadalapaka G, et al. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene 2013;32:1616-25. [Crossref] [PubMed]
- Pang EJ, Yang R, Fu XB, et al. Overexpression of long non-coding RNA MALAT1 is correlated with clinical progression and unfavorable prognosis in pancreatic cancer. Tumour Biol 2015;36:2403-7. [Crossref] [PubMed]
- An M, Lohse I, Tan Z, et al. Quantitative Proteomic Analysis of Serum Exosomes from Patients with Locally Advanced Pancreatic Cancer Undergoing Chemoradiotherapy. J Proteome Res 2017;16:1763-72. [Crossref] [PubMed]
- Que R, Ding G, Chen J, et al. Analysis of serum exosomal microRNAs and clinicopathologic features of patients with pancreatic adenocarcinoma. World J Surg Oncol 2013;11:219. [Crossref] [PubMed]
- Madhavan B, Yue S, Galli U, et al. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int J Cancer 2015;136:2616-27. [Crossref] [PubMed]
- Richards KE, Zeleniak AE, Fishel ML, et al. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene 2017;36:1770-8. [Crossref] [PubMed]
- Kisiel JB, Raimondo M, Taylor WR, et al. New DNA Methylation Markers for Pancreatic Cancer: Discovery, Tissue Validation, and Pilot Testing in Pancreatic Juice. Clin Cancer Res 2015;21:4473-81. [Crossref] [PubMed]
- Yi JM, Guzzetta AA, Bailey VJ, et al. Novel methylation biomarker panel for the early detection of pancreatic cancer. Clin Cancer Res 2013;19:6544-55. [Crossref] [PubMed]
- Nones K, Waddell N, Song S, et al. Genome-wide DNA methylation patterns in pancreatic ductal adenocarcinoma reveal epigenetic deregulation of SLIT-ROBO, ITGA2 and MET signaling. Int J Cancer 2014;135:1110-8. [Crossref] [PubMed]
- Falco A, Rosati A, Festa M, et al. BAG3 is a novel serum biomarker for pancreatic adenocarcinomas. Am J Gastroenterol 2013;108:1178-80. [Crossref] [PubMed]
- Radon TP, Massat NJ, Jones R, et al. Identification of a Three-Biomarker Panel in Urine for Early Detection of Pancreatic Adenocarcinoma. Clin Cancer Res 2015;21:3512-21. [Crossref] [PubMed]
- Zhong Y, Naito Y, Cope L, et al. Functional p38 MAPK identified by biomarker profiling of pancreatic cancer restrains growth through JNK inhibition and correlates with improved survival. Clin Cancer Res 2014;20:6200-11. [Crossref] [PubMed]
- Baliyan V, Kordbacheh H, Parakh A, et al. Response assessment in pancreatic ductal adenocarcinoma: role of imaging. Abdom Radiol (NY) 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Choi M, Heilbrun LK, Venkatramanamoorthy R, et al. Using 18F-fluorodeoxyglucose positron emission tomography to monitor clinical outcomes in patients treated with neoadjuvant chemo-radiotherapy for locally advanced pancreatic cancer. Am J Clin Oncol 2010;33:257-61. [PubMed]
- Ramanathan RK, Goldstein D, Korn RL, et al. Positron emission tomography response evaluation from a randomized phase III trial of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone for patients with metastatic adenocarcinoma of the pancreas. Ann Oncol 2016;27:648-53. [Crossref] [PubMed]