
A novel scoring system to predict ascites development post hepatectomy for BCLC stage B hepatocellular carcinoma
Introduction
The annual mortality of hepatocellular carcinoma (HCC) is over 625,000 worldwide. It is the 5th most common cancer type and more than 50% of the cases occurred in china (1,2). Among them, nearly 85% patients had history of chronic hepatitis or cirrhosis (3). Ascites, as one of the hallmarks of the portal hypertension and advanced cirrhosis, is also a common manifestation of HCC (4). American Association for the Study of Liver Diseases(AASLD) (5) and European Association for the Study of the Liver (EASL) (6) had developed guidelines to provide evidence-based recommendations for the assessment and treatment of ascites. As aggressive surgical resection is the main treatment for HCC in China and many countries in Asia, ascites is one of common complications post hepatectomy, and this can result in serious complications leading to adverse outcome (7,8). Comparing with the ascites caused by hepatitis or cirrhosis, post-operative ascites is different in etiology, clinical manifestation and management. To our knowledge, there is no standard guideline focusing on the prevention and treatment of ascites post hepatectomy as it might be more complicated with lack of high level of evidence to guide clinical decision. Controversy still exists in clinical practice with resultant remarkable difference in management and outcome from center to center (9-11). It is extremely important to identify those patients at risk of developing post-operative ascites early, so intervention can be implemented to improve the outcome. Ascites were more likely occurred in BCLC stage B HCC than in stage A because of the larger impacts of liver resection (12). Many known clinicopathological factors are related to the development of post-operative ascites. However, more research is needed to better understand those factors and their potential use in prediction role, especially for the patient of BCLC stage B HCC who were at high risk in perioperative time. In this analysis, we studied various predictive factors related to development of post-operative ascites in 181 consecutive BCLC stage B HCC patients who underwent hepatectomy in our center from January 2005 to December 2014. Based on this, we created a scoring system that can stratify patients into different risk groups of developing post-operative ascites.
Methods
Patients
Subjects included 181 BCLC stage B (single tumor >5 cm, or multi nodular) (13) HCC patients who had undergone hepatectomy at Beijing Cancer Hospital, China, between January 2005 and December 2014. Patients were excluded if they had been previously treated with trans arterial chemoembolization (TACE), radiofrequency ablation (RFA), or radiotherapy. All tumors were confirmed by pathology and staged according to the AJCC Cancer Staging Manual, 7th edition. All patients with reported history of hepatitis B were confirmed by hepatitis B surface antigen (HBsAg) test using enzyme-linked immunosorbent assay. Six patients with hepatitis B had co-infection of hepatitis C virus (HCV). All patients were diagnosed with cirrhosis by a history of chronic liver disease, characteristic findings from computed tomography (CT), magnetic resonance imaging (MRI) or ultrasonography, and was confirmed by surgical findings and pathology. All patients had no evidence of distal metastases. This study protocol was in accord with the Declaration of Helsinki and subsequent amendments and had been approved by the institutional review board in our hospital. Written informed consent from all patients was obtained before enrollment.
Definition of post-operative ascites
We defined the post-operative ascites when it met one of following criteria: (I) daily ascites drainage >500 mL, at least one day post operation; (II) the total volume of drainage >1,500 mL in 7 days post operation; (III) the free peritoneal fluid >500 mL confirmed by ultrasound, CT or MRI. Patients were excluded if drainage contained obvious bile, blood, digestive juice, pancreatic juice or chyle.
Diagnosis and treatment of HCC
Diagnosis of HCC was made by laboratory findings, image findings from ultrasonography, CT and/or MRI, and tissue diagnosis if necessary, all in accordance with the guidelines of the EASL (14) and AASLD (15). Intraoperative ultrasound was used in all patients during surgery to seek additional tumors. Liver parenchymal transection was performed primarily using the clamp crushing method or Cavitron Ultrasonic Surgical Aspirator (CUSA) (16) with intermittently Pringle’s inflow occlusion. Hepatic resection was usually performed under low central venous pressure anesthesia in order to minimize the bleeding (17). Abdominal drains were left with cut surface and connected to a closed drainage system. During the operation, fresh frozen plasma (FFP) was transfused at a rate that exceeded the amount of blood loss by 10%. Intraoperative red blood cell (RBC) transfusion was given only if blood loss exceeded 800 mL or the hemoglobin decreased to less than 8 g/L.
Statistical analysis
Pre-identified risk factors were compared between post-operative ascites group and non-ascites group using t-test, chi-square test, Fisher’s exact test or Wilcoxon rank-sum test wherever appropriate. A best predictive model was identified by incorporating risk factors and covariates into a logistic model and using a backward variable selection method with an alpha level of removal at 0.1. The ability of the prediction model to estimate the risk of developing ascites was assessed using the area under the receiver operating characteristic (ROC) curve. All analyses were performed using SAS 9.3 software (SAS Institute, Inc., Cary, NC, USA). All statistical tests were two-tailed, and a P value of less than 0.05 was considered statistically significant.
Results
Ascites was identified in 62 (34.3%) patients post hepatectomy including 48 (77.4%) male and 14 (22.6%) female. Ascites was more often present in female (14/25, 56.0%) than in male (48/156, 30.8%, P<0.05) patients, as shown in Table 1. The median age of ascites group was 51.4 years (range, 29.0–78.0 years), which was similar to the non-ascites group [50.4 years, (range, 27.0–78.0 years), P>0.05]. In addition, gender, prothrombin time (PT), prothrombin activity (PTA), platelet count (PLT), aspartate transaminase (AST) were different with statistical significance (P<0.05) between ascites group and non-ascites group.
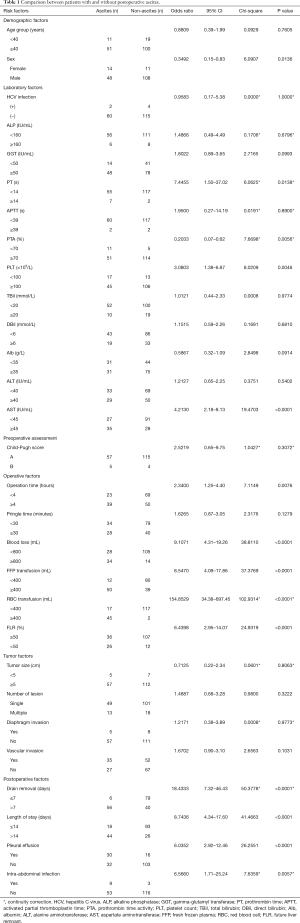
Full table
The median operation time was 3.9 hours (range, 1.2–8.5 hours). The ascites group had longer operation time than the non-ascites group (P<0.01). However, the Pringle time had no impact on ascites accumulation when 30 minutes was used as the cut-off. Blood loss and RBC transfusion were higher in the ascites group than in the non-ascites group. The patients with postoperative ascites also had larger volume of intraoperative FFP transfusion than the patients without ascites. In total, 26/38 (68.4%) patients with future liver remnant (FLR) less than 50% developed ascites after major hepatectomy, while only 36/143 (25.2%) patients with FLR larger than 50% had ascites.
The median postoperative length of stay was 15.7 days (range, 6.0–66.0 days). The ascites group had statistically significant (P<0.01) longer length of stay than the non-ascites group. Drain placement time was also longer with ascites. In total, 30/62 (48.4%) patients in ascites group developed pleural effusion, whereas 16/119 (13.4%) patients did in non-ascites group. Among 12 patients diagnosed with intra-abdominal infection by positive culture of peritoneal fluid, 9 had post-operative ascites.
Multivariate analysis revealed that PLT, RBC transfusion, FFP transfusion and FLR independently affected the risk of postoperative ascites accumulation. A novel scoring model was thus developed to predict the development of ascites using the regression coefficients of the multivariate model (Table 2).
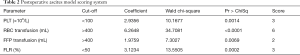
Full table
The PLT less than 100×109/L, RBC transfusion greater than 400 mL, FFP transfusion greater than 400 mL, and FLR less than 50% were given the scores of 3, 6, 2, 3, respectively. ROC curve analysis was used to evaluate the sensitivity and specificity of the scores for all patients. It revealed that the patients with scores ≥5 had high risk of developing post-operative ascites [sensitivity =96.8%, specificity =90.8%, and area under the curve (AUC) =0.972] (Figure 1).
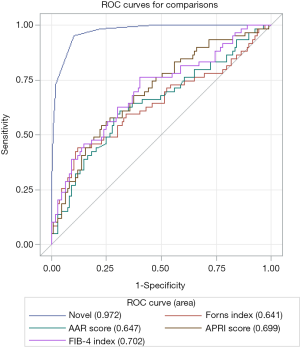
To further evaluate the efficacy and usefulness of this novel model, we compared it with previously reported fibrotic scores (AAR score, APRI score, Forns index and FIB-4 index). The AAR score is the ratio between the AST and the ALT (expressed as IU/L) (18). The APRI score is the ratio between the number of times above the upper limit of normal (ULN) of AST and the PLT (109/L) (19). The Forns index is calculated using the following formula: 7.811−3.131× ln [PLT count (109/L)] +0.781× ln [r-GT (IU/L)] +3.467× ln [age (years)] −0.014× cholesterol (mg/dL) (20). The FIB-4 index is calculated as age (years) × AST (IU/mL)/PLT (109/L) × ALT (IU/mL)1/2 (21). The area under the ROC curve (AUROC, 95% CI) was greatest with our new model (0.972, 0.951–0.992), followed by the FIB-4 index (0.702, 0.617–0.787), APRI score (0.699, 0.615–0.782), AAR score (0.647, 0.557–0.738) and Forns score (0.641, 0.546–0.735) (Figure 1).
Discussion
Ascites is one of the most common complications post hepatectomy for HCC. The incidence of ascites post hepatectomy is reported between 15% and 56% and is associated with poorer prognosis (22-24). There are consensuses and guidelines for assessment and management of ascites induced by liver fibrosis or cirrhosis (25,26), but is paucity of data pertaining ascites post hepatectomy for treatment of HCC. For those patients, using the grading of EASL guidelines is not optimal due to the abdominal drainage, which underestimated the severity of the ascites (6,27). In addition, there is no general consensus for the definition of the postoperative ascites. Ishizawa et al. defined significant ascites as postoperative daily fluid drainage from both thorax and peritoneal cavity exceeding 10 mL/kg of preoperative body weight (23). However, in another study, the postoperative ascites was defined as daily ascites fluid drainage exceeding 500 mL and/or EASL grade 2 ascites by ultrasonography or clinical assessment with finding of moderately symmetrical distension of the abdomen (24). In our study, we also introduced an index, which is the total volume of ascites fluid drainage for 7 days post operation, this may better suit for situations such as temporary obstruction of the drainage, which led to the daily ascites fluid drainage less than the cut-off of 500 mL. In addition, we removed certain subjective variables that cannot be quantified.
The underlying mechanism of developing post hepatectomy ascites has not been well established. In cirrhotic patients, it was postulated that the increasing resistance to portal flow at the sinusoidal level results in the development of sinusoidal portal hypertension and the backward transmission of this increased pressure into splanchnic capillaries (28). Massive ascites can be induced by stimulating neurohormonal system with resultant enhancement of renal water and sodium reabsorption in patients with portal hypertension. Furthermore, as the hepatic vascular bed was decreased after liver resection, portal hypertension may reduce renal blood flow and urinary output during the early postoperative period and subsequently increase the volume of ascites (29). Chan et al. retrospectively analyzed 651 HCC patients who underwent liver resection and identified that the development of postoperative ascites was associated with cirrhosis, high indocyanine green retention, portal hypertension, hypoalbuminemia, and the extent of liver resection (24). Ishizawa et al. retrospectively analyzed 203 HCC patients who underwent hepatectomy, blood loss and preoperative PLT were found be related to the increased risk of developing postoperative ascites (23). In another Chinese study with 73 patients studied, Indocyanine green retention rate at 15 minutes (ICGR15) >10%, tumor size >10 cm and RBC transfusion were identified in multivariate analysis (30). Overall, development of postoperative ascites may be associated with many clinicopathological characteristics. This can make it very difficult to correctly predict the incidence or the extent of the ascites (31). We previously published a cohort of 324 HCC patients who underwent liver resection. Retrospective analysis revealed six variables that were related to development of post-operative ascites, those were PLT, AST, intraoperative plasma transfusion, hemihepatectomy or extended hemi-hepatectomy, the urine output and the drainage amount in postoperative day 1. A predictive scoring system was subsequently developed using all six variables to predict postoperative ascites, the specificity and the sensitivity were 86.2% and 83.3%, respectively (32). However, that study included both BCLC stage A and BCLC stage B patients. As reported, BCLC stage B patients were more likely to present ascites postoperatively than BCLC stage A (12). Therefore, it is necessary to stratify patients based on BCLC stage for the analysis of post-operative ascites.
Previous studies had reported of using fibrotic score to predict the development of post-operative ascites. It was a reasonable approach as advanced liver fibrosis or cirrhosis is significantly related to an increased risk of hepatic decompensation and ascites development (33). However, the area under the ROC curve of using fibrotic score was remarkably lower than our current novel scoring system. This might indicate that the fibrosis or cirrhosis were not the only factors that impact the incidence of the ascites. Other factors associated with operation should also be taken into account.
There are limitations of our study. Transient elastography (FibroScan) is one of the noninvasive tools to predicts ascites after liver resection for HBV-related HCC (34). Our study did not include the liver stiffness measurement value using FibroScan and the pathological fibrosis score of American Joint Committee on Cancer (AJCC, 7th edition, 2010). Therefore, the usefulness of those measurements in predicting post-operative ascites in our study population is unknown. Another limitation of this study is that only HBV-related HCC patients were recruited due to the high prevalence of HBV infection which led to more than 80% HCC in China (35). Therefore, our results cannot be generalized to patients with HCC caused by etiologies other than HBV. It is also unknown whether high HBV DNA level may contribute to the development of postoperative ascites. Further study is needed to study this relationship.
From our study, we demonstrated that the development of postoperative ascites was associated with multiple factors that include the pre-operative baseline liver condition, the process of the operation and also the intensity of perioperative blood product support. Precise and comprehensive analysis of these factors is needed to further identify the etiology of post-operative ascites and to guide the development of strategies to prevent and manage this complication following hepatectomy. Our novel scoring system, if validated, could provide a possible tool to predict the post-operative ascites after hepatectomy and hence direct the appropriate clinical management.
Acknowledgments
Funding: This work was supported by Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (approval No.: XMLX201708) and International Science & Technology Cooperation Program of China (approval No.: 2013DFG32720).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.01.35). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study protocol was in accord with the Declaration of Helsinki and subsequent amendments and had been approved by the Institutional Review Board of Peking University Cancer Hospital. Written informed consent from all patients was obtained before enrollment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-76. [Crossref] [PubMed]
- Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006;45:529-38. [Crossref] [PubMed]
- Gines P, Schrier RW. Renal failure in cirrhosis. N Engl J Med 2009;361:1279-90. [Crossref] [PubMed]
- Runyon BA. AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology 2013;57:1651-3. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010;53:397-417. [Crossref] [PubMed]
- Zhang XF, Meng B, Qi X, et al. Prognostic factors after liver resection for hepatocellular carcinoma with hepatitis B virus-related cirrhosis: surgeon's role in survival. Eur J Surg Oncol 2009;35:622-8. [Crossref] [PubMed]
- Jin S, Fu Q, Wuyun G, et al. Management of post-hepatectomy complications. World J Gastroenterol 2013;19:7983-91. [Crossref] [PubMed]
- Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg 2003;138:1198-206; discussion 2206. [Crossref] [PubMed]
- Taketomi A, Kitagawa D, Itoh S, et al. Trends in morbidity and mortality after hepatic resection for hepatocellular carcinoma: an institute's experience with 625 patients. J Am Coll Surg 2007;204:580-7. [Crossref] [PubMed]
- Kusano T, Sasaki A, Kai S, et al. Predictors and prognostic significance of operative complications in patients with hepatocellular carcinoma who underwent hepatic resection. Eur J Surg Oncol 2009;35:1179-85. [Crossref] [PubMed]
- Gomaa AI, Al-Khatib A, Abdel-Razek W, et al. Ascites and alpha-fetoprotein improve prognostic performance of Barcelona Clinic Liver Cancer staging. World J Gastroenterol 2015;21:5654-62. [Crossref] [PubMed]
- Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010;30:61-74. [Crossref] [PubMed]
- European Association For The Study Of The Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. Erratum in: J Hepatol 2012;56:1430. [Crossref] [PubMed]
- Bruix J, Sherman MAmerican Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Lesurtel M, Belghiti J. Open hepatic parenchymal transection using ultrasonic dissection and bipolar coagulation. HPB (Oxford) 2008;10:265-70. [Crossref] [PubMed]
- Rahbari NN, Koch M, Zimmermann JB, et al. Infrahepatic inferior vena cava clamping for reduction of central venous pressure and blood loss during hepatic resection: a randomized controlled trial. Ann Surg 2011;253:1102-10. [Crossref] [PubMed]
- Cheung RC, Currie S, Shen H, et al. Can we predict the degree of fibrosis in chronic hepatitis C patients using routine blood tests in our daily practice? J Clin Gastroenterol 2008;42:827-34. [Crossref] [PubMed]
- Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011;53:726-36. [Crossref] [PubMed]
- Forns X, Ampurdanes S, Llovet JM, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002;36:986-92. [Crossref] [PubMed]
- Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317-25. [Crossref] [PubMed]
- Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut 2006;55:vi1-12. [Crossref] [PubMed]
- Ishizawa T, Hasegawa K, Kokudo N, et al. Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg 2009;144:46-51. [Crossref] [PubMed]
- Chan KM, Lee CF, Wu TJ, et al. Adverse outcomes in patients with postoperative ascites after liver resection for hepatocellular carcinoma. World J Surg 2012;36:392-400. [Crossref] [PubMed]
- Moore KP, Wong F, Gines P, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology 2003;38:258-66. [Crossref] [PubMed]
- Gines P, Cardenas A, Arroyo V, et al. Management of cirrhosis and ascites. N Engl J Med 2004;350:1646-54. [Crossref] [PubMed]
- Donadon M, Costa G, Cimino M, et al. Safe hepatectomy selection criteria for hepatocellular carcinoma patients: a validation of 336 consecutive hepatectomies. The BILCHE score. World J Surg 2015;39:237-43. [Crossref] [PubMed]
- Sussman AN, Boyer TD. Management of refractory ascites and hepatorenal syndrome. Curr Gastroenterol Rep 2011;13:17-25. [Crossref] [PubMed]
- Moreau R, Gaudin C, Hadengue A, et al. Renal hemodynamics in patients with cirrhosis: relationship with ascites and liver failure. Nephron 1993;65:359-63. [Crossref] [PubMed]
- Chen LP, Li C, Wang C, et al. Risk factors of ascites after hepatectomy for patients with hepatocellular carcinoma and hepatitis B virus-associated cirrhosis. Hepatogastroenterology 2012;59:292-5. [Crossref] [PubMed]
- Huo TI, Lui WY, Wu JC, et al. Deterioration of hepatic functional reserve in patients with hepatocellular carcinoma after resection: incidence, risk factors, and association with intrahepatic tumor recurrence. World J Surg 2004;28:258-62. [Crossref] [PubMed]
- Wei M, Qian HG, Qiu H, et al. A scoring system to predict ascites after hepatectomy for hepatocellular carcinom. Zhonghua Wai Ke Za Zhi 2010;48:1534-8. [PubMed]
- Kim SU, Lee JH, Kim DY, et al. Prediction of liver-related events using fibroscan in chronic hepatitis B patients showing advanced liver fibrosis. PLoS One 2012;7:e36676 [Crossref] [PubMed]
- Li C, Zhang JY, Zhang XY, et al. FibroScan predicts ascites after liver resection for hepatitis B virus-related hepatocellular carcinoma: A prospective cohort study. Int J Surg 2015;20:21-5. [Crossref] [PubMed]
- Liu CJ, Kao JH. Hepatitis B virus-related hepatocellular carcinoma: epidemiology and pathogenic role of viral factors. J Chin Med Assoc 2007;70:141-5. [Crossref] [PubMed]


