
Prognostic factors and outcomes of osseous chondrosarcoma after surgery: the 2004–2014 Surveillance, Epidemiology, and End Results database study
Introduction
Chondrosarcoma is a slow-growing malignant tumor comprised of transformed cells producing a cartilaginous matrix (1,2), it occurs common at skeletal system, and also could be at extraskeletal sites (3,4). The prognostic factors that affect the survival outcomes were inconsistent in previous studies (5-9) and still lack the data of patients with osseous chondrosarcoma after surgery.
In last 10 years, many osseous chondrosarcoma after surgery patients were registered to the Surveillance, Epidemiology, and End Results (SEER) database. Moreover, after 2004, the information of grade, American Joint Committee on Cancer (AJCC) staging system stage, tumor size were more completion than pervious (almost half information of the grade, tumor size and stage of osseous chondrosarcoma was unavailable before 2004).
The aim of present study is to use the 2004–2014 SEER databases to investigate the prognostic factors and survival outcomes of chondrosarcoma osseous chondrosarcoma after surgery.
Methods
Patients diagnosed with chondrosarcoma (histological type ICD-O-3: “9220/3: Chondrosarcoma, not otherwise specified’’) are searched using the case-listing session protocol of the National Cancer Institute’s SEER 18 databases (www.seer.cancer.gov) (10). Only the patients registered at 2004–2014 are included in present study. The patients don’t perform the surgery or the tumors sited at extraskeletal tissues are excluded.
We extract the demographic information include: age, gender, race, year of diagnosis, tumor sites, tumor size, grade, stages (AJCC staging system). The stages are classified to three types (11,12): (I) localized: tumor confined to cortex of bone or extension beyond cortex but confined within periosteum; (II) regional: extension beyond periosteum to surrounding tissues including adjacent skeletal muscle, adjacent bone/cartilage, or skin; or (III) distal metastasis. This article does not contain any identified human participants of the SEER database.
Statistical analysis
The age is converted to a categorical variables of 0–44, 45–59, 60–74, ≥75 years old, Tumor sites are concluded into four of limbs, vertebral column, pelvis/sacrococcyx, and other bones (such as skull, rib, sternum, clavicle, and mandible). The grades of poorly differentiated and undifferentiated are combined by the similar type and small sample of undifferentiated. Multivariable Cox proportional hazard regression models are used to calculate the HRs with 95% CIs for chondrosarcoma cancer-specific survival (CCSS). The patients with lost data are excluded when perform the Multivariable Cox proportional hazard regression models. All statistical analysis is performed using STATA software (Version 14.2; Stata Corp, College Station, TX, USA).
Results
Total of 1,630 osseous chondrosarcoma patients performed surgery are included in present study. The characteristics of included patients are summarized in Table 1. The age at the diagnosis is 50.9±17.2 years old. And the percentage of male is 53.07%, slight higher than the female (46.93%). Most of them are White population (87.91%), the Black and other race is 6.13% and 4.85%, respectively. The follow-up term is 53.5±37.5 months.

Full table
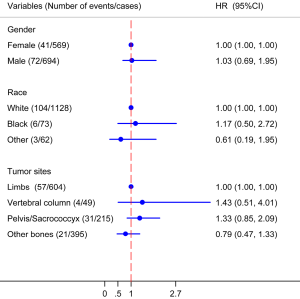
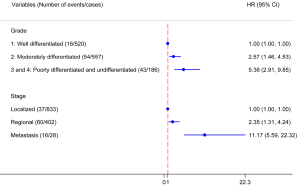
The Multivariable Cox proportional hazard regression models find the factors of gender, race and tumor sites have no significant associated with the CCSS (Figure 1). However, the grades of moderately differentiated, “poorly differentiated and undifferentiated” have poorer outcomes when the well differentiated osseous chondrosarcoma used as reference, with HR (95% CI) of 2.57 (1.46–4.53) and 5.36 (2.91–9.85), respectively. The stages of regional and metastasis also have poorer outcomes when the localized osseous chondrosarcoma used as reference, with HR (95% CI) of 2.35 (1.31–4.24) and 11.17 (5.59–22.32), respectively (Figure 2).
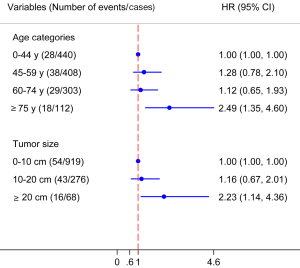
No significant difference are observed among the age categories of 0–44, 45–59, 60–74 years and tumor size categories of 0–10 and 10–20 cm by multivariable Cox proportional hazard regression models test. But patients have age ≥75 years, and tumor size ≥20 cm have poorer outcomes than categories (Figure 3).
Discussion
In present study, only the patients registered after 2004 are included. Because before the 2004, the most information of grade, tumor size, and stage of chondrosarcoma is lost and unavailable, it will influence the credible of results. After 2004, the AJCC staging system 6th edition (13) is used at SEER, and information is more completion. Moreover, considering the surgical types are very varies before 2000, and have more consistent after then (14,15), therefore, we use the data after 2004.
Additional, compared to the previous study of Giuffrida et al. (9), only the osseous chondrosarcoma are included, because of the heterogonous sites of extraskeletal chondrosarcoma make the heterogonous survival rates (16). Some histological subtypes of chondrosarcoma, such as dedifferentiated (17) and mesenchymal (18) variants are generic term for a diverse group of skeletal sarcomas, also not included in this study. Only the histological type ICD-O-3: “9220/3: Chondrosarcoma, not otherwise specified’’ is included to make the consistent of single disease.
In studies of Marcove et al. (6), Gitelis et al. (7), and Lee et al. (8), only the grade was found as a prognostic factor for chondrosarcoma, but not the stage. In studies of Rizzo et al. (5), grade was also not found as a prognostic factor. The reason may the sample in their study is too small. In our present study, most current 1,630 patients data find both the grade and stage are the independent prognostic factors. The higher grade and stage have significant poorer survival than the lower grade and stage, the HRs are increased ladder-like as the grade and stage increased (Figure 2).
We also classify the age to categories of 0–44, 45–59, 60–74, ≥75 years, and tumor size to categories of 0–10, 10–20 and ≥20 cm. We find the survival outcomes don’t have line trends with age and tumor size. The patients with age less than 75 years have similar survival outcomes and only the patients ≥75 years have the poorer survival outcomes. Similar results are observed in tumor size, only patients with tumor size more than 20 cm have the poorer outcomes, which is not find in previous studies (5,6,8,9).
The strengths of our present study include: (I) most recently [2004–2014] and with large sample size of 1,630 cases; (II) the multivariable analysis of CCSS included variables of age, gender, race, year of diagnosis, tumor sites, tumor size, grade, stages; (III) the included patients are consistently.
There are some limitations of present study, the SEER database has it nature drawbacks of don’t combine some detailed information of patient comorbidities and adjuvant treatments, lack of central radiologic and pathologic review by experts. And this is an observational study, may have some cofounders can’t be included.
Conclusions
We found grade, stage are independent prognostic factors for survival rate of osseous chondrosarcoma after surgery, and higher age more than 75 years, bigger tumor size more than 20 cm is also predicted poor outcomes.
Acknowledgments
Funding: This work was funded by the National Natural Science Foundation of China [81501933, 81572214], Zhejiang Provincial Natural Science Foundation of China (LY14H060008), Zhejiang Provincial Medical and Health Technology Foundation of China (2018KY129), Wenzhou leading talent innovative project (RX2016004) and Wenzhou Municipal Science and Technology Bureau (Y20170389).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.03.14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Review by the institutional review board was not required for this study as the SEER database is publicly available without individually identifiable private information. Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gelderblom H, Hogendoorn PC, Dijkstra SD, et al. The clinical approach towards chondrosarcoma. Oncologist 2008;13:320-9. [Crossref] [PubMed]
- Sundaresan N, Rosen G, Boriani S. Primary malignant tumors of the spine. Orthop Clin North Am 2009;40:21-36. v. [Crossref] [PubMed]
- Jakowski JD, Wakely PE Jr. Cytopathology of extraskeletal myxoid chondrosarcoma: report of 8 cases. Cancer 2007;111:298-305. [Crossref] [PubMed]
- Sadashiva N, Sharma A, Shukla D, et al. Intracranial Extraskeletal Mesenchymal Chondrosarcoma. World Neurosurg 2016;95:618.e1-6. [Crossref] [PubMed]
- Rizzo M, Ghert MA, Harrelson JM, et al. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res 2001;224-33. [Crossref] [PubMed]
- Marcove RC, Miké V, Hutter RV, et al. Chondrosarcoma of the pelvis and upper end of the femur. An analysis of factors influencing survival time in one hundred and thirteen cases. J Bone Joint Surg Am 1972;54:561-72. [Crossref] [PubMed]
- Gitelis S, Bertoni F, Picci P, et al. Chondrosarcoma of bone. The experience at the Istituto Ortopedico Rizzoli. J Bone Joint Surg Am 1981;63:1248-57. [Crossref] [PubMed]
- Lee FY, Mankin HJ, Fondren G, et al. Chondrosarcoma of bone: an assessment of outcome. J Bone Joint Surg Am 1999;81:326-38. [Crossref] [PubMed]
- Giuffrida AY, Burgueno JE, Koniaris LG, et al. Chondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER database. J Bone Joint Surg Am 2009;91:1063-72. [Crossref] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program Research Data (1973-2014). Available online: https://seer.cancer.gov/data/
- Mukherjee D, Chaichana KL, Adogwa O, et al. Association of extent of local tumor invasion and survival in patients with malignant primary osseous spinal neoplasms from the surveillance, epidemiology, and end results (SEER) database. World Neurosurgery 2011;76:580-5. [Crossref] [PubMed]
- Arshi A, Sharim J, Park DY, et al. Chondrosarcoma of the Osseous Spine: An Analysis of Epidemiology, Patient Outcomes, and Prognostic Factors Using the SEER Registry From 1973 to 2012. Spine (Phila Pa 1976) 2017;42:644-52. [Crossref] [PubMed]
- Greene FL, Page DL, Fleming ID, et al. editors. 6th edition. AJCC cancer staging manual. New York: Springer Science & Business Media, 2002.
- Gutowski CJ, Basu-Mallick A, Abraham JA. Management of bone sarcoma. Surg Clin North Am 2016;96:1077-106. [Crossref] [PubMed]
- Chow WA. Update on chondrosarcomas. Curr Opin Oncol 2007;19:371-6. [Crossref] [PubMed]
- Kemmerer EJ, Gleeson E, Poli J, et al. Benefit of Radiotherapy in Extraskeletal Myxoid Chondrosarcoma: A Propensity Score Weighted Population-based Analysis of the SEER Database. Am J Clin Oncol 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Bovée JV, Cleton-Jansen AM, Rosenberg C, et al. Molecular genetic characterization of both components of a dedifferentiated chondrosarcoma, with implications for its histogenesis. J Pathol 1999;189:454-62. [Crossref] [PubMed]
- Fanburg-Smith JC, Auerbach A, Marwaha JS, et al. Reappraisal of mesenchymal chondrosarcoma: novel morphologic observations of the hyaline cartilage and endochondral ossification and β-catenin, Sox9, and osteocalcin immunostaining of 22 cases. Hum Pathol 2010;41:653-62. [Crossref] [PubMed]


