
Successful ablation for pulmonary artery tumor thrombosis more than 5 cm with massive hepatocellular carcinoma and multiple pulmonary metastases
Introduction
The prognosis of advanced hepatocellular carcinoma (HCC) patients with extrahepatic disease (EHD) and vascular invasion remains dismal (1,2). Pulmonary artery has been reported to be a common tumor-involved vessel in lung metastasis from HCC (3) which indicates a reduction of survival. Pulmonary artery tumor invasion contributes significantly to the poor survival of advanced HCC patients.
The reported strategies, including surgical resection, chemotherapy, and radiotherapy, which are performed for the lung metastasis of HCC, may be effective to prevent pulmonary artery tumor thrombosis (PATT) from progressing, but the high risk of intra-operative thromboembolism, respiratory failure, post-operative complications and treatment-related toxicity should be taken into account seriously.
Percutaneous thermal ablation is helpful for the management of advanced HCC with vascular invasion. Thermal ablation energy produces high temperature, which results in tumor necrosis via cellular dehydration and protein denaturation. Previous studies (4-7) have reported the safety and effectiveness of radiofrequency ablation (RFA) for advanced HCC with portal vein thrombus, and the 2-year survival rate is between 77% and 78.8% with no severe adverse events. Besides, our experience in treating HCC extending into portal vein or right atrium also shows that thermal ablation is an alternative approach to prevent the progression of tumor thrombosis (8,9). However, the feasibility and safety of RFA for PATT originated from a massive HCC have been rarely reported.
We reported a rare and difficulty case of PATT from massive HCC with multiple lung metastases, and complete tumor response was achieved after RFA.
Case presentation
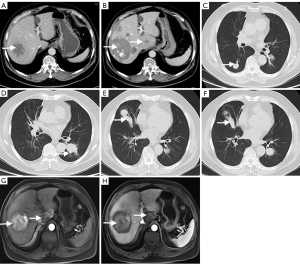
A 72-year-old man with history of right-lobe massive HCC of 8.3 cm in diameter (Figure 1A), a caudate lobe HCC of 5.5 cm in diameter (Figure 1B), multiple bilateral pulmonary metastases (PM) (Figure 1C,D) and coronary atherosclerosis was admitted to our hospital for PATT (Figure 1E,F) in July, 2016.
Pre-operative laboratory examination included the following: Chile-Pugh grade, A; MELD score, 7; alpha-fetoprotein (AFP), 2.49 ng/mL; CA19-9, 30.81 ng/mL; prothrombin time (PT), 10.5 seconds; PT activity, 114%; leukocyte (WBC), 8.72×109/L; hemoglobin (HGB), 154 g/L; blood platelet (PLT), 236×109; FQ-HBV-DNA, 4.58×105 copies/mL; HBsAg (+); anti-HBs (−); HBeAg (−); anti-HBe (+); anti-HBc (+); HCV-IgG (−); alanine aminotransferase (ALT), 28.1 U/L; aspartate transaminase (AST), 18.4 U/L; total bilirubin (TBiL), 13.9U/L; albumin (ALB), 44.2g/L.
In the initial hospitalization (October, 2011), a large mass of 8.3 cm in diameter in the liver was found via contrasted-enhanced computed tomography (CECT). The mass showed hypervascular enhancement during the hepatic arterial phase and hypoattenuation during venous phase. Furthermore, ultrasound-guided fine needle biopsy confirmed the diagnosis of HCC. In February 2014, the HCC located in caudate lobe was found by CECT. During the period between October 2011 and February 2014, 6 sessions of ablation were performed for HCCs, and the latest contrast-enhanced magnetic resonance imaging (MRI) of abdomen showed an ablation zone of low attenuation in arterial phase (Figure 1G,H) which was defined as complete response.
In July 2016, CT scan of the chest detected recurrent lung metastases in the right lobe and left lobe, and a massive embolus of 5.8 cm in diameter in the right pulmonary artery. Pre-operative pathological examination through fine needle biopsy was guided by ultrasound. The pathological examination of the thrombosis revealed malignant tumorous tissue and confirmed the diagnosis of PATT originating from HCC.
The patient was assessed in the multidisciplinary consultation as having advanced-stage HCC with lung metastases and PATT, and RFA was recommended.
Informed written signed consent was obtained before each procedure.
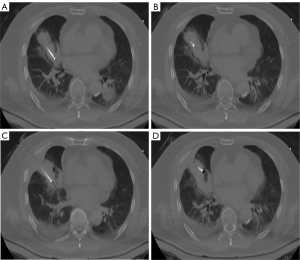
During the RFA procedures for PATT, the patient was under local anesthesia with continuous monitoring of vital signs. All procedures were under CT guidance (AquililionTM, Toshiba Medical CO, Tokyo, Japan). The best puncture path to reach the thrombosis should avoid injuring significant structures. The patient was placed in the supine decubitus position. A 22-G Chiba needle (COOK, Bloomington, IN, USA) was advanced into the target lesion, leading the RFA electrode (VIVA, STARmed, Goyang, Korea) to the target. The angle and depth of each puncture was calculated based on intraoperative CT scans to ensure that the needle was in the correct position and direction. First, the part with proximity to heart was ablated, using an output power of 100 W for 12 minutes (Figure 2A). Then, the needle was withdrawn along the long axis of PATT to ablate the stem with an output power of 150 W for 10 minutes. The branches of PATT was then ablated using an output power of 100 W for 10 minutes (Figure 2B,C,D). Finally, the needle track from the tumor to the percutaneous puncture point was ablated in order to prevent tumor cell dissemination and bleeding. An instant CT scan without contrast medium was obtained to evaluate whether the technique is successful and to detect complications. There were no serious immediate complications such as hemorrhage, pneumothorax, and the target tumor was necrotic completely. The post-procedural enhanced CT scan on forth day revealed that the PATT was surrounded by exudation zone (Figure 3A,B) which was induced by RFA. Ten days after ablation, the patient was discharged.
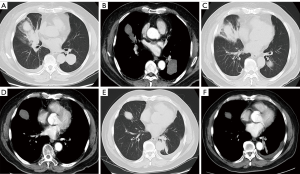
CECT was done during routine follow-up. Four months later, the CECT showed that the PATT had shrunk significantly and no enhancement in arterial phase or wash-out findings in venous phase was detected in the ablative area, which indicated complete necrosis of the PATT (Figure 3C,D). During follow-up, at 3-month interval, PATT was under control. Except 3 sessions of microwave ablation were carried out for the pulmonary metastases located in left lobe, no other treatment was performed during the follow-up period for the PATT. There was no progression and recurrence of PATT at the 14-month follow-up (Figure 3E,F).
The patient who is still alive has survived 6 years after the beginning of initial treatment course of primary HCC, and 14 months after the treatment of PATT.
Discussion
This report presented a successful case of treating PATT with multiple lung metastases originated from HCC by RFA.
The prevalence of tumor pulmonary artery tumor embolism is between 0.05% and 0.56%, and is more frequent in HCC with an occurrence rate of 0.34% (10). The incidence of pulmonary tumor embolism is higher through autopsy, which is between 3% and 26% (11). It is one of the manifestations of advanced HCC associated with a poor prognosis. The survival of patients with PATT without any treatment was no more than 12 weeks (12). Furthermore, PATT could cause sudden death (13).
The treatment strategies of vascular tumor thrombosis are controversial. A case report presented a fibroblastic osteosarcoma patient with PATT which is treated by chemotherapy combined with lobectomy, and the patient survived 1 year (14). However, the treatment-related toxicity overstepped patients’ tolerance and induced interruption of treatment. Masaki et al. (15) reported a 48-year-old HCC patient with PATT who only received open-heart surgery, and the tumor embolus was extracted from the pulmonary artery successfully. However, the patient died during hospitalization and the survival after resection was only 29 days. Embolectomy and inferior vena cava filter placement could prolong the survival of PATT patient to 30 months (16). The survival after surgery seems promising; however, it should be noted that fragments of tumor emboli produced by surgery could increase the risk of tumor embolism via venous or lymphatic drainage (12). Besides, general anesthesia, a median sternotomy, cardiopulmonary bypass and a longitudinal incision from the obstructed artery trunk are necessary elements of surgical procedures; but these are intolerant for patients with poor general condition, particularly for old patients. In this report, the patient was intolerant for an open approach, and the concurrent disease also limited the tolerance of surgery.
Percutaneous RFA is a promising therapy for HCC with vascular invasion. Traditional theory (17) claims that the procedures of RFA performed in blocked vessel are difficult, however, several published studies showed promising results. Lazoura et al. (18) developed an arterial luminal stenosis model which disclosed the safety and feasibility of endovascular application of RF. Mizandari et al. (6) used RFA to treat portal vein tumor embolus without any specific complications. Li et al. (8) reported a promising survival of 16 months after ablation for HCC with tumor embolus entering the right atrium and inferior vena cava; however, the median survival after supportive treatment and surgical treatment were only 5 and 6 months (19-21). Hence, percutaneous thermal ablation, particularly RFA, appears to be a safe and effective approach for tumor thrombosis. The application of RF energy in PATT, was theoretically feasible, and was complete successful in our procedure with a more promising survival than surgical treatment (15).
During RFA, following key points were necessary: First, the puncture path must avoid bullae and major pulmonary vessels. The needle should enter along the long axis of PATT until reaching the proximal end of the PATT adjacent to heart. Second, since the thrombosis block the pulmonary artery, it is not suggested to puncture directly through the vascular wall, due to a high risk of massive hemorrhage. A more operable path is puncturing the tumor thrombosis through pulmonary metastasis lesion. The metastases could be easily ablated subsequently after the thrombosis has been ablated, and this would lessen the number of punctures and reduce the risk of bleeding and technique failure. Third, during procedures, the parameter of ablation device should be set carefully to avoid injuring adjacent vital structures, and a single-needle is recommended.
The potential benefit of RFA treating PATT are as follow: the RF energy could destroy the tumor embolus and prevent dissemination; complete tumor response after RFA could reduce the pressure of pulmonary artery and the possibility of sudden death; it is possible to downstage the primary cancer intending to meet the criteria of resection, transplantation or RFA.
In conclusion, we performed a successful ablation preventing the PATT from progressing, and prolonged the survival of the patient. Further studies are warranted to validate the therapeutic efficacy of ablation for advanced HCC patients with PATT.
Acknowledgments
Funding: This study is supported by Beijing Talents Project; Funding for High-level Talents in Beijing Municipal Health System (grant number 2014-3-088); National Major Scientific Instruments and Equipment Development Project (grant number ZDYZ2015-2); Beijing Natural Science Foundation (grant number 7142078); National Twelve-Five Key Technology Support Program (grant number 2012BAI15B08); Beijing Youan Hospital Hepatic Disease & HIV Fund (grant number 20150203) and the National Natural Science Foundation of China (grant number H1617/ 81472328).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.03.24). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hou JZ, Zeng ZC, Zhang JY, et al. Influence of tumor thrombus location on the outcome of external-beam radiation therapy in advanced hepatocellular carcinoma with macrovascular invasion. Int J Radiat Oncol Biol Phys 2012;84:362-8. [Crossref] [PubMed]
- Kim SU, Kim YR, Kim DY, et al. Clinical features and treatment outcome of advanced hepatocellular carcinoma with inferior vena caval invasion or atrial tumor thrombus. Korean J Hepatol 2007;13:387-95. [Crossref] [PubMed]
- Nagayasu T. Importance of follow-up inspection after pulmonary angioplastic procedures for lung cancer surgery. Gen Thorac Cardiovasc Surg 2010;58:1-2. [Crossref] [PubMed]
- Giorgio A, Di Sarno A, de Stefano G, et al. Hepatocellular carcinoma with cirrhosis: are patients with neoplastic main portal vein invasion eligible for percutaneous radiofrequency ablation of both the nodule and the portal venous tumor thrombus? AJR Am J Roentgenol 2009;193:948-54. [Crossref] [PubMed]
- Hirooka M, Koizumi Y, Kisaka Y, et al. Mass reduction by radiofrequency ablation before hepatic arterial infusion chemotherapy improved prognosis for patients with huge hepatocellular carcinoma and portal vein thrombus. AJR Am J Roentgenol 2010;194:W221-6 [Crossref] [PubMed]
- Mizandari M, Ao G, Zhang Y, et al. Novel percutaneous radiofrequency ablation of portal vein tumor thrombus: safety and feasibility. Cardiovasc Intervent Radiol 2013;36:245-8. [Crossref] [PubMed]
- Zhang L, Yan J, Liu F, et al. Experimental study on the safety of percutaneous transhepatic portal vein ablation. Hepatogastroenterology 2015;62:126-32. [PubMed]
- Li W, Wang Y, Gao W, et al. HCC with tumor thrombus entering the right atrium and inferior vena cava treated by percutaneous ablation. BMC Surg 2017;17:21. [Crossref] [PubMed]
- Long J, Zheng JS, Sun B, et al. Microwave ablation of hepatocellular carcinoma with portal vein tumor thrombosis after transarterial chemoembolization: a prospective study. Hepatol Int 2016;10:175-84. [Crossref] [PubMed]
- Sakuma M, Fukui S, Nakamura M, et al. Cancer and pulmonary embolism: thrombotic embolism, tumor embolism, and tumor invasion into a large vein. Circ J 2006;70:744-9. [Crossref] [PubMed]
- Roberts KE, Hamele-Bena D, Saqi A, et al. Pulmonary tumor embolism: a review of the literature. Am J Med 2003;115:228-32. [Crossref] [PubMed]
- Winterbauer RH, Elfenbein IB, Ball WC Jr. Incidence and clinical significance of tumor embolization to the lungs. Am J Med 1968;45:271-90. [Crossref] [PubMed]
- Chan GS, Ng WK, Ng IO, et al. Sudden death from massive pulmonary tumor embolism due to hepatocellular carcinoma. Forensic Sci Int 2000;108:215-21. [Crossref] [PubMed]
- Buderi S, Theologou T, Gosney J, et al. Pulmonary artery tumor embolism in a patient with previous fibroblastic osteosarcoma. Ann Thorac Surg 2013;95:2155-7. [Crossref] [PubMed]
- Masaki N, Hayashi S, Maruyama T, et al. Marked clinical improvement in patients with hepatocellular carcinoma by surgical removal of extended tumor mass in right atrium and pulmonary arteries. Cancer Chemother Pharmacol 1994;33:S7-11. [Crossref] [PubMed]
- Daughtry JD, Stewart BH, Golding LA, et al. Pulmonary embolus presenting as the initial manifestation of renal cell carcinoma. Ann Thorac Surg 1977;24:178-81. [Crossref] [PubMed]
- Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36. [Crossref] [PubMed]
- Lazoura O, Zacharoulis D, Kanavou T, et al. A novel experimental animal model of arterial stenosis based on endovascular radiofrequency energy application. J Invest Surg 2011;24:123-8. [Crossref] [PubMed]
- Luo X, Zhang B, Dong S, et al. Hepatocellular Carcinoma With Tumor Thrombus Occupying the Right Atrium and Portal Vein: A Case Report and Literature Review. Medicine (Baltimore) 2015;94:e1049 [Crossref] [PubMed]
- Miller DL, Katz NM, Pallas RS. Hepatoma presenting as a right atrial mass. Am Heart J 1987;114:906-8. [Crossref] [PubMed]
- Wang Y, Yuan L, Ge RL, et al. Survival benefit of surgical treatment for hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: results of a retrospective cohort study. Ann Surg Oncol 2013;20:914-22. [Crossref] [PubMed]


