
Hypofractionation in prostate cancer radiotherapy
Introduction
There is much debate amongst historians about who was first to use ionizing radiation for the treatment of cancer, with various claims from both sides of the Atlantic. By contrast, there is general agreement that radiotherapeutic practice during the first two decades of the 20th century was dominated by the German School at Erlangen, which advocated the use of a single “castrating” dose of X-rays (1). Considering the relatively primitive equipment available at the time, it is not surprising that the clinical results were poor. By the 1930’s animal experiments at the Institute Curie in Paris suggested that spreading the dose over a period of several weeks could result in better tumor control for a given normal tissue toxicity. This was followed by extensive clinical studies on the effects of treatment duration, culminating with publications from Coutard showing results for the treatment of various head and neck tumors that were clearly superior to the German data (2). This set the general pattern for radiotherapy over much of the world for the best part of 50 years, with conventional wisdom requiring 20 to 40 dose fractions over a period of 3 to 6 weeks. The basis of fractionation in radiotherapy can be understood in simple terms. Dividing a dose into several fractions spares normal tissues because of repair of sublethal damage between dose fractions as well as repopulation of cells if the overall time is sufficiently long. At the same time, dividing a dose into several fractions increases damage to the tumor because of reoxygenation and reassortment of cells into radiosensitive phases of the cycle between dose fractions. The advantages of prolongation of treatment are to spare early reactions and to allow adequate reoxygenation in tumors. Excessive prolongation, however, allows surviving tumor cells to proliferate during treatment.
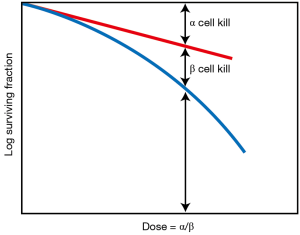
A landmark discovery in radiation biology in the 1980’s was the recognition of a systematic difference between the fractionation dependence of acute and late reacting normal tissues (3). Late reactions are much more dependent on the size of the dose fraction than are acute reactions. In terms of the linear-quadratic relationship between dose and effect, this translates into a larger α/β ratio for early effects than for late effects. The α/β ratio is the dose at which cell killing by the linear (α) and the quadratic (β) components are equal. This is illustrated in Figure 1. For early effects, the α/β ratio is large; as a consequence, alpha dominates at low doses, so that the dose-response curve has a marked initial slope and does not bend until higher doses. The linear and quadratic components of cell killing are not equal until about 10 Gy. As a consequence, fractionation of the dose results in only a modest decrease in biological effect. By contrast, for late effects, the α/β ratio is small, so that the beta term has an influence at low doses and the dose—response curve bends at lower doses to appear more curved; the linear and quadratic components of cell killing are equal at about 2 Gy. As a consequence, fractionating the dose results in a significant sparing of the biological effect. This difference between early and late responding tissues is illustrated in Figure 2.
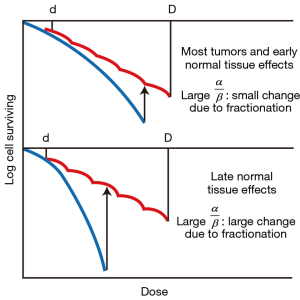
This understanding of a difference between early responding tissues (and most tumors) compared with late responding tissues led to the fashion of the 1980’s and 1990’s to increase the number of dose fractions to amplify the difference in response of late responding normal tissues compared with tumors; i.e., better tumor control with less normal tissue toxicity. Clinical trials were performed with as many as 70 dose fractions, only possible by planning two fractions per day, separated by 6 hours (4). Clinical trials showed the superiority of hyperfractionation, as it was called, for several tumor sites, confirming the validity of the radiobiological evidence. However, hyperfractionated radiotherapy never became mainstream because of the difficulty of scheduling multiple treatments per day in a busy radiation oncology department.
Hypofractionation for prostate cancer radiotherapy
In 1999, Brenner and Hall suggested (5) that the accepted argument that multiple dose fractions would reduce toxicity to late-responding tissues for a given tumor control may not necessarily apply to prostate cancer, possibly because prostate tumors often grow so slowly. They suggested a method to calculate the α/β ratio for prostate cancer by comparing published data for external beam radiotherapy with comparable data for brachytherapy. They found an α/β ratio for prostate cancer of about 1.5 Gy, comparable for that for late responding normal tissues, and much smaller than for most other tumors (5). While the reason for to difference in α/β ratios between early and late tissues has never been conclusively proven, a plausible hypothesis is that the α/β ratio of a tissue is determined by the proportion of cycling cells compared with cells that are not dividing. Because most prostate tumors are slow growing there is not a major differential in terms of cell division rates between a typical prostate tumor and the surrounding late responding normal tissue. This finding removes the advantage conferred by multiple dose fractions, and led to the suggestion that “if the fractionation sensitivity is the same for the tumor and the surrounding late-responding normal tissue, much smaller numbers of fractions (with an appropriately reduced dose) would be expected to be at least as efficacious, but logistically and financially advantageous.” This technique of using smaller numbers of larger dose fractions has come to be known as prostate cancer hypofractionation.
Later in the same year, Duchesne and Peters (6) independently questioned the magnitude of the α/β ratio for prostate cancer, and concluded that there was strong circumstantial evidence for a low α/β ratio similar to that for late-responding normal tissue, and argued that “the use of hypofractionated brachytherapy may, in fact, be beneficial rather than merely expedient, and may increase the therapeutic ratio for treatment of clinically localized prostate cancer.”
Since these two papers were published in 1999 there have been two principal developments: first, there have been a number of independent attempts to estimate the value of the α/β ratio for prostate cancer from clinical data. These are summarized in Table 1 (7-15). The results, despite sometimes wide confidence limits, are generally much lower than the typical values (16) for other types of tumors, supporting the original hypothesis (5) that prostate cancer responds to fractionation more like a late-responding tissue rather than an early responding tissue. Although there is no conclusive evidence—despite hints in the reports by Valdagni et al. (17) and Pollack et al. (18,19)—it might well be expected that advanced aggressive prostate cancers may not have such low α/β ratios, in which case hypofractionation may not be appropriate for such cases.

Full table
Second, investigators across the globe have set out to design prospective trials to test the hypofractionation hypothesis in prostate cancer. Initially non randomized trials, and more recently randomized trials have been reported (19-28), some with only around a hundred patients but more recently several with 1,000 to 3,000 patients. These randomized trials are based on two quite different objectives: Some are designed to show that hypofractionation gives results as good as conventional therapy, i.e., a “non-inferiority” design. The advantage of hypofractionation would then be convenience and economic. Other trials are designed to show that hypofractionation gives better results than the conventional fractionation, either improved tumor control or reduced morbidity or both, a “superiority” design.
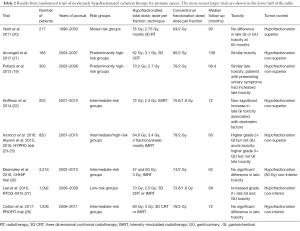
Table 2 summarizes the results of eight randomized clinical trials that have been published (19-28) of moderately hypofractionated radiation therapy for prostate cancer. Moderately hypofractionated is defined here as using between 2.5 and 3.5 Gy per fraction. The trials vary considerably in size, with some accumulating several hundred patients while others involve thousands of subjects. The trials also vary considerably in regard to the hypofractionated dose and dose per fraction used. The four earlier smaller randomized studies are at the top of Table 2, with the four later, larger randomized studies in the lower half of the table.
Full table
Focusing on the four larger randomized trials, the PROFIT trial (28) and the CHHiP trial (26) both used 3 Gy fractions, the CHHiP trial using a total dose of either 57 or 60 Gy, and the PROFIT trial using 60 Gy, with median follow up of 5 or 6 years, respectively. Both of these large studies showed that hypofractionation was non-inferior in regard to tumor control, and showed no significant differences in terms of late sequelae. The RTOG-0415 study (27) used a lower dose per fraction (2.5 Gy), but a much larger total dose (70 Gy), and had a median followup of 69 months; as with the PROFIT and CHHiP trials, the RTOG-0415 study demonstrated non-inferiority for hypofractionation in terms of tumor control, but there was an increase in grade 2+ late GI and GU toxicity. Finally the HYPRO trial (23-25) used a significantly higher dose per fraction (3.4 Gy) and total dose (64.6 Gy) as compared with the PROFIT and CHHiP trials; the HYPRO trial showed non superiority of the hypofractionation arm in term of tumor control, but also showed higher grade 2+ GI (but not GU) acute toxicity and higher grade 3+ genitourinary (GU) [but not gastrointestinal (GI)] toxicity; these toxicity increases can be understood in terms of the high dose per fraction and dose that were used in the HYPRO file.
Two specific observations are in order: (I) of the four large studies, the only one to show significant increased normal tissue toxicity used the largest dose per fraction; (II) the only study that showed significant superiority (as opposed to non-inferiority) for hypofractionation in terms of tumor control was the trial by Yeoh et al. (20). However there are several caveats; first, it was a comparatively small study with only 217 patients, and second the comparability in terms of risk levels between the hypofractionated and conventional arms may have been questionable (20).
Overall, while non-inferiority of hypofractionation in terms of tumor control now seems well established, it is evident that the “superiority trials”, based on the hypothesis that moderate hypofractionation would increase tumor control efficacy compared with conventional fractionation, have produced negative results; however this was to be expected since, in retrospect, it was an over interpretation of the original hypothesis to expect hypofractionation to be superior to conventional fractionation. To quote from the original paper (5) “Appropriately designed hypofractionation regimes would be expected to maintain current levels of tumor control and late sequelae, but with reduced acute morbidity, together with the logistic and financial advantages of fewer number of fractions”. With the possible exception of reduced acute morbidity—which seems to be largely the same—these 1999 predictions have been born out by the subsequent randomized clinical trials. Indeed, it may now be possible to suggest evidence-based guidelines for moderate hypofractionated regimens (29).
Extreme hypofractionation for prostate cancer radiotherapy
All the discussions above have referred to so called “moderate” hypofractionation which is usually defined as involving dose per fraction of 2.5 to 3.5 Gy, and fraction numbers of at least 15. There have also been a number of trials reported in which still smaller number of fractions, 4 to 12, have been used with large doses per fraction of 4 to 10 Gy—so called extreme hypofractionation. Such protocols have been made possible through the advantageous dose distributions produced by stereotactic body radiation therapy (SBRT), or robotic radiosurgery, or proton therapy. The early clinical results to date from extreme fractionation have been reviewed by Höcht et al. (30). In summary, while the tumor control has been excellent, moderate to high grade acute toxicity has typically been very high, ranging from 10% to 20%. Most of the extreme hypofractionation studies are not yet mature enough to report late sequelae (31).
It is important to emphasize that the rationale for the use extreme hypofractionation is not based solely on the low α/βratio characteristic of prostate cancer. Such treatments are made possible because of technological advances in dose delivery that enable the delivery of a very large dose of radiation to a tumor with reduced margins and a high dose gradient outside the target area. As a consequence, the volume of normal tissue exposed to high doses of radiation is greatly reduced. These highly conformal techniques have already shown impressive results for the lung and brain, and are considered particularly suitable to treat small tumors embedded in a normal tissue where the functional subunits are in parallel (32). However in the context of prostate radiotherapy, both the rectum and the bladder respond, at least in large part, as serial organs (33,34).
Most of the extreme hypofractionation studies for prostate cancer reported to date were not randomized (30). Early results from a phase 2 randomized study of extreme hypofractionation have been reported by Lukka et al. (35) (RTOG-0938, 5×7.25 vs. 12×4.3 Gy/fraction; 1 year follow-up), and from a phase III study by Widmark et al. (36)(HYPO-RT-PC, 7×6.1 Gy/fraction; 2 year follow-up). These, and several other non-randomized extreme hypofractionation studies, show early promise, but of course both tumor control and normal tissue toxicities remain to be fully assessed. In this regard, a number of phase III trials of extreme hypofractionation are ongoing, focusing specifically on use of just 5 fractions: The doses per fraction vary from 7.25 (37) to 7.6 Gy (38) and up to as high as 8 Gy (39). Dose delivery is highly conformal in these studies, either through the use of SBRT (37,39) or with proton therapy (38). The two issues that hopefully will be addressed in these trials are (I) tumor control: in particular a concern is whether as few as 5 fractions will be sufficient to overcome tumor hypoxia (40), and (II) late sequelae: in particular whether the excellent dose distributions used in these studies will be sufficient to limit late sequelae from these quite aggressive—at least in terms of biologically effective dose (29)—protocols. These two, as yet answered, questions lie at the heart of the potential utility of extreme hypofractionation for prostate cancer.
Conclusions
Prostate cancer hypofractionation represents a pleasing example of successful translational cancer research. The initial suggestions for moderate prostate hypofractionation (5,6) grew out of a hard-won mechanistic understanding (3) of the fundamental basis of fractionation in radiotherapy, and progressed—after much debate in the literature—to non-randomized and now randomized clinical trials. Moderate hypofractionation now seem likely to become standard of care in prostate cancer radiotherapy. The potential utility of extreme hypofractionation is not, however, yet established.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Israel Deutsch, James McKiernan, Charles Drake) for the series “Prostate Cancer: Current Understanding and Future Directions” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.01.30). The series “Prostate Cancer: Current Understanding and Future Directions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wintz H. Die Einzeitbestrahlung. Strahlentherapie 1937;58:545-51.
- Coutard H. The Results and Methods of Treatment of Cancer by Radiation. Ann Surg 1937;106:584-98. [Crossref] [PubMed]
- Thames HD Jr, Withers HR, Peters LJ, et al. Changes in early and late radiation responses with altered dose fractionation: implications for dose-survival relationships. Int J Radiat Oncol Biol Phys 1982;8:219-26. [Crossref] [PubMed]
- Horiot JC, Le Fur R, N'Guyen T, et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: final analysis of a randomized trial of the EORTC cooperative group of radiotherapy. Radiother Oncol 1992;25:231-41. [Crossref] [PubMed]
- Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 1999;43:1095-101. [Crossref] [PubMed]
- Duchesne GM, Peters LJ. What is the a/b ratio for prostate cancer? Rationale for hypofractionated high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 1999;44:747-8. [PubMed]
- Brenner DJ, Martinez AA, Edmundson GK, et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low a/b ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 2002;52:6-13. [Crossref] [PubMed]
- Wang JZ, Guerrero M, Li XA. How low is the a/b ratio for prostate cancer? Int J Radiat Oncol Biol Phys 2003;55:194-203. [Crossref] [PubMed]
- Bentzen SM, Ritter MA. The alpha/beta ratio for prostate cancer: what is it, really? Radiother Oncol 2005;76:1-3. [Crossref] [PubMed]
- Williams SG, Taylor JM, Liu N, et al. Use of individual fraction size data from 3756 patients to directly determine the alpha/beta ratio of prostate cancer. Int J Radiat Oncol Biol Phys 2007;68:24-33. [Crossref] [PubMed]
- Proust-Lima C, Taylor JM, Secher S, et al. Confirmation of a low alpha/beta ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys 2011;79:195-201. [Crossref] [PubMed]
- Miralbell R, Roberts SA, Zubizarreta E, et al. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: alpha/beta = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys 2012;82:e17-24. [Crossref] [PubMed]
- Vogelius IR, Bentzen SM. Meta-analysis of the alpha/beta ratio for prostate cancer in the presence of an overall time factor: bad news, good news, or no news? Int J Radiat Oncol Biol Phys 2013;85:89-94. [Crossref] [PubMed]
- Pedicini P, Strigari L, Benassi M. Estimation of a self-consistent set of radiobiological parameters from hypofractionated versus standard radiation therapy of prostate cancer. Int J Radiat Oncol Biol Phys 2013;85:e231-7. [Crossref] [PubMed]
- Boonstra PS, Taylor JM, Smolska-Ciszewska B, et al. Alpha/beta (alpha/beta) ratio for prostate cancer derived from external beam radiotherapy and brachytherapy boost. Br J Radiol 2016;89:20150957 [Crossref] [PubMed]
- Thames HD, Bentzen SM, Turesson I, et al. Fractionation parameters for human tissues and tumors. Int J Radiat Biol 1989;56:701-10. [Crossref] [PubMed]
- Valdagni R, Nahum AE, Magnani T, et al. Long-term biochemical control of prostate cancer after standard or hyper-fractionation: evidence for different outcomes between low-intermediate and high risk patients. Radiother Oncol 2011;101:454-9. [Crossref] [PubMed]
- Pollack A, Walker G, Horwitz EM, et al. J Clin Oncol 2014;32:1853-4. [Crossref] [PubMed]
- Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol 2013;31:3860-8. [Crossref] [PubMed]
- Yeoh EE, Botten RJ, Butters J, et al. Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: final results of phase III randomized trial. Int J Radiat Oncol Biol Phys 2011;81:1271-8. [Crossref] [PubMed]
- Arcangeli G, Saracino B, Arcangeli S, et al. Moderate Hypofractionation in High-Risk, Organ-Confined Prostate Cancer: Final Results of a Phase III Randomized Trial. J Clin Oncol 2017;35:1891-7. [Crossref] [PubMed]
- Hoffman KE, Voong KR, Pugh TJ, et al. Risk of late toxicity in men receiving dose-escalated hypofractionated intensity modulated prostate radiation therapy: results from a randomized trial. Int J Radiat Oncol Biol Phys 2014;88:1074-84. [Crossref] [PubMed]
- Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2016;17:1061-9. [Crossref] [PubMed]
- Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol 2016;17:464-74. [Crossref] [PubMed]
- Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): acute toxicity results from a randomised non-inferiority phase 3 trial. Lancet Oncol 2015;16:274-83. [Crossref] [PubMed]
- Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 2016;17:1047-60. [Crossref] [PubMed]
- Lee WR, Dignam JJ, Amin MB, et al. Randomized Phase III Noninferiority Study Comparing Two Radiotherapy Fractionation Schedules in Patients With Low-Risk Prostate Cancer. J Clin Oncol 2016;34:2325-32. [Crossref] [PubMed]
- Catton CN, Lukka H, Gu CS, et al. Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J Clin Oncol 2017;35:1884-90. [Crossref] [PubMed]
- Brenner DJ, Hall EJ. Are we now able to define guidelines for moderate hypofractionation in prostate cancer radiotherapy? Int J Radiat Oncol Biol Phys 2018;100:871-3. [Crossref] [PubMed]
- Höcht S, Aebersold DM, Albrecht C, et al. Hypofractionated radiotherapy for localized prostate cancer. Strahlenther Onkol 2017;193:1-12. [Crossref] [PubMed]
- Koontz BF, Bossi A, Cozzarini C, et al. A systematic review of hypofractionation for primary management of prostate cancer. Eur Urol 2015;68:683-91. [Crossref] [PubMed]
- Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol 2014;32:2847-54. [Crossref] [PubMed]
- Fiorino C, Rancati T, Valdagni R. Predictive models of toxicity in external radiotherapy: dosimetric issues. Cancer 2009;115:3135-40. [Crossref] [PubMed]
- Zhu J, Simon A, Haigron P, et al. The benefit of using bladder sub-volume equivalent uniform dose constraints in prostate intensity-modulated radiotherapy planning. Onco Targets Ther 2016;9:7537-44. [Crossref] [PubMed]
- Lukka H, Stephanie P, Bruner D, et al. Patient-Reported Outcomes in NRG Oncology/RTOG 0938, a Randomized Phase 2 Study Evaluating Two Ultra-hypofractionated Regimens (UHRs) for Prostate Cancer. Int J Radiat Oncol Biol Phys 2016;94:2. [Crossref]
- Widmark A, Gunnlaugsson A, Beckman L, et al. Extreme Hypofractionation versus Conventionally Fractionated Radiotherapy for Intermediate Risk Prostate Cancer: Early Toxicity Results from the Scandinavian Randomized Phase III Trial “HYPO-RT-PC”. Int J Radiat Biol Oncol Phys 2016;96:938-9. [Crossref]
Prostate Advances in Comparative Evidence (PACE) - Vargas C. Study of Hypo-fractionated Proton Radiation for Low Risk Prostate Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01230866
- Zelefsky M. Trial of ADT and SBRT Versus SBRT for Intermediate Prostate Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03056638
- Brown JM, Diehn M, Loo BW Jr. Stereotactic ablative radiotherapy should be combined with a hypoxic cell radiosensitizer. Int J Radiat Oncol Biol Phys 2010;78:323-7. [Crossref] [PubMed]


