Efficacy and safety of apatinib in patients with metastatic colorectal cancer refractory to standard therapies
Introduction
Colorectal cancer (CRC) is the third most common cancer and the fourth most common cause of cancer-related death worldwide (1). Twenty-five percent of patients with CRC have metastases which in turn have a detrimental effect on prognosis (2,3). Patients with mCRC are often treated with chemotherapeutic agents, including fluoropyrimidine, oxaliplatin and irinotecan; which may be combined with targeted therapy such as bevacizumab, cetuximab/panitumumab (4). After all standard treatments failing, patients may be treated with regorafenib or TAS102, but regorafenib was not approved in China until May 6, 2017 and TAS102 has not approved until now. Therefore, there is an urgent need to develop an effective and safe therapeutic approach for the treatment of mCRC, especially for Chinese patients.
Angiogenesis is an essential step in tumor growth and metastasis. Vascular endothelial growth factor (VEGF) signaling has an important role in angiogenesis, while vascular endothelial growth factor receptors (VEGFRs) function as key regulators in this process (5). The VEGFR family of proteins consist of VEGFR-1 [FMS-like tyrosine kinase (FLT)-1], VEGFR-2 (KDR/Flk-1), and VEGFR-3 (FLT-4). VEGFR-2 is the principal mediator of VEGF-induced angiogenic signaling (6), and thus targeting VEGFR-2 represents a promising strategy for inhibiting tumor-induced angiogenesis and tumor growth.
As a small-molecule tyrosine kinase inhibitor (TKI), apatinib binds to VEGFR-2, inhibiting VEGF binding and subsequent VEGFR-2 auto-phosphorylation (7). In addition, apatinib-mediated VEGFR-2 inhibition leads to the inhibition of downstream phosphorylated extracellular signal-regulated kinase (p-ERK), resulting in antiangiogenic and antitumor effects (7). Apatinib also targets c-Kit, Ret, and c-Src (7).
Apatinib was approved and launched in China in 2014 as a third- or fourth-line treatment for patients with advanced gastric cancer (8). Existing clinical experiments have also indicated that apatinib has potential as a therapeutic agent for the treatment of non-small cell lung cancer, and breast cancer (9,10). In the present study, we evaluated the efficacy and safety of apatinib in patients with refractory mCRC. We hoped that our results would help to develop a novel targeted therapy for mCRC.
Methods
Patient eligibility
Patients with pathologically confirmed metastatic or recurrent colorectal adenocarcinoma were treated with apatinib between August 2015 and April 2017. All patients were resistant or intolerable to conventional chemotherapeutic agents, including fluoropyrimidines, oxaliplatin, or irinotecan with or without anti-VEGFs or anti-EGFRs agents. The performance status of patients ranged within 0–2 at the time of starting the apatinib treatment.
Treatment methods
With a treatment cycle of 28 days (4 weeks), each patient orally received 500 mg of apatinib once a day until disease progression, death, unacceptable toxic effects, withdrawal of consent by the patient, or decision by the treating physician that discontinuation would be in the best interest of the patient. One dose reduction (to 250 mg) for drug-related grade 3 or 4 toxicity was allowed.
Responses and toxicity
Tumor response and progression were initially assessed every four weeks, and after two or three cycles, they were assessed at 8-week interval using RECIST version 1.1 (11). Overall response rate (ORR) and disease control rate (DCR) were defined as the proportion of patients with complete response (CR) and partial response (PR), and the sum of patients with CR, PR and stable disease (SD) in the population, respectively. Progression-free survival (PFS) was defined as the interval from the initiation of apatinib to the date of tumor progression or death from any cause. Overall survival (OS) was defined as the time from the first day of apatinib treatment to death or last follow-up. Adverse events (AE) were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (CTC4.0) (12).
Statistical analysis
Statistical analysis was performed using SPSS version 21.0 (SPSS Inc., Chicago, Illinois, USA). The survival analysis was conducted by the Kaplan-Meier analysis and comparison analysis by the log-rank test. P<0.05 was regarded as statistically significant.
Results
Patient characteristics
Forty-one patients were included in this retrospective study; among these, 26 were male and 15 were female with a median age of 55 years. Twenty-eight patients had a PS of 1. Twenty-two patients had primary tumor sites at the colon and 19 at the rectum. Thirty-four patients had the operation of primary tumors. Eleven patients had more than three organs with metastatic diseases. KRAS wide-type was present in 21 patients. Prior to the treatment with apatinib, all patients received at least two lines of chemotherapy, consisting of fluoropyrimidine (41/41, 100%), irinotecan (40/41, 97.6%), or oxaliplatin (40/41, 97.6%). Moreover, the treatments for the 30 patients were combined with target therapies. The patient characteristics are listed in Table 1.
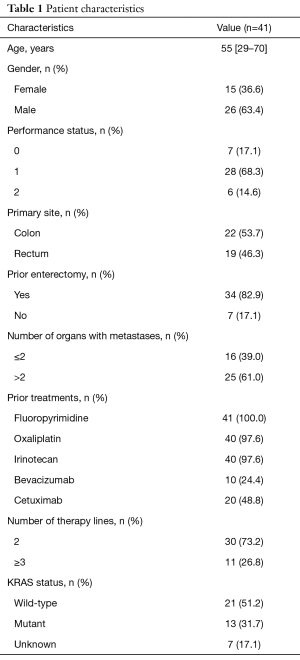
Full table
Efficacy
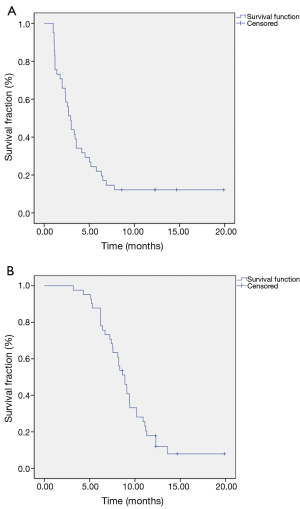
The best objective response to apatinib was PR in 4 (9.8%) patients and SD in 27 (65.9%) patients, respectively. The ORR was 9.8% and DCR was 75.6%. The median PFS (mPFS) was 2.9 months (95% CI, 2.4–3.5) while median OS (mOS) was 8.9 months (95% CI, 8.0–9.8) (Figure 1).
Thirty-one patients were never treated with anti-angiogenesis before using apatinib. The median PFS and OS in these patients were 3.2 months (95% CI, 2.3–4.1) and 9.1 months (95% CI, 7.5–10.6), respectively. The ORR was 9.7% and the DCR was 77.4%.
There was no significant association between PFS or OS and age, gender, PS, tumor site, operation history, metastases organ numbers and lines of therapy (Table 2).
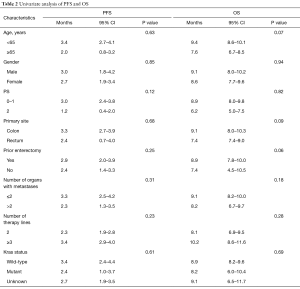
Full table
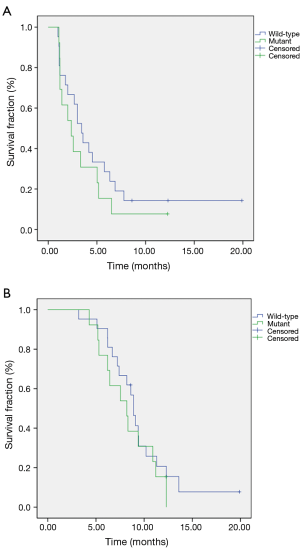
Efficacies in Kras wild-type group compared to the group with Kras mutant were mPFS of 3.4 vs. 2.4 months (HR =0.54; 95% CI, 0.52–1.80; P=035), and mOS of 8.9 vs. 8.2 months (HR =0.62; 95% CI, 0.32–1.54; P=0.41), respectively (Figure 2).
Safety
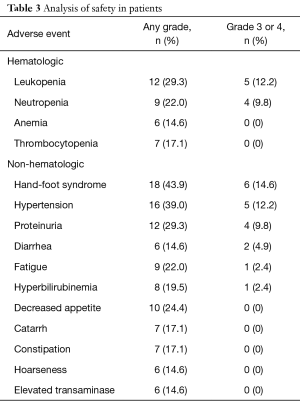
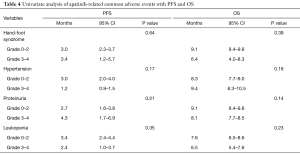
All patients initially received a daily oral dose of 500 mg of apatinib. However, the dose was reduced to 250 mg in one patient due to recurrent grade 3 hypertension. The common hematologic AEs caused by apatinib among these patients were leukopenia (12/41, 29.3%), neutropenia (9/41, 22.0%), anemia (6/41, 14.6%), and thrombocytopenia (7/41, 17.1%). The common non-hematologic toxicities were hypertension (16/41, 39.0%), hand-foot syndrome (HFS; 18/41, 43.9%), proteinuria (12/41, 29.3%) and fatigue (9/41, 22.0%). The grade 3/4 toxicities were HFS (6/41, 14.6%), hypertension (5/41, 12.2%), leukopenia (5/41, 12.2%), proteinuria (4/41, 9.8%), neutropenia (4/41, 9.8%), diarrhea (2/41, 4.9%), fatigue (1/41, 2.4%) and hyperbilirubinemia (1/41, 2.4%) (Table 3). There was no statistically significant PFS or OS with apatinib-related common AEs: HFS, hypertension, proteinuria and leukopenia (Table 4).
Full table
Full table
Discussion
To the best of our knowledge, this is the first study to report the efficacy and safety of apatinib as salvage therapy in mCRC.
Fluorouracil-based chemotherapy, which is widely used in routine mCRC treatment and targeted therapy, including anti-EGFR and anti-VEGF therapy, has demonstrated improved outcomes for mCRC (13,14). Nevertheless, these patients had very limited options if the abovementioned therapies failed. Regorafenib has been approved for the salvage treatment of patients with advanced and heavily treated CRC based on two phase III randomized controlled trials (15,16). Nonetheless, it was not approved in China until May 6, 2017. Under such circumstances, we used apatinib for treating patients with mCRC who were refractory to standard therapies.
It has been reported that a 500 mg/day dose of apatinib is effective in pretreated metastatic triple-negative breast cancer (TNBC), with encouraging rates of SD and PFS; and that most AEs were mild to moderate in severity (grades 1–2) (17). In the present study, considering that most of the patients had declining performance status (PS ≥1, 82.9%) after two or more lines of therapies, we used 500 mg of apatinib as the starting dose.
In the present study, the median PFS and OS in patients was 2.9 and 8.9 months, which were observed to be similar to that of regorafenib (3.2 and 8.8 months) in the CONCUR trial (16). There were 31 patients who were never treated with anti-angiogenesis before using apatinib. The mOS of these patients seemed to be better than regorafenib reported by Riechelmann in 2018 ASCO GI (18). We think that use of anti-angiogenesis before maybe a factor affecting the efficacy of apatinib.
In univariate analysis, all parameters were not statistically significant, but we found that the mOS seemed to have a prolonged trend in patients younger than 65 years old or patients with primary site located in the colon or those having enterectomy. We think that more cases are needed to determine whether these factors are associated with better prognosis.
Numerous studies have proved that the tumor mutational status is one of the important determinants of the response of metastatic colorectal cancer (mCRC) to targeted treatments. In the present study, we did not find the different survival in patients treated with apatinib accordingly to their K-ras status (wild-type/mutation) which was similar to Regorafenib (15). However, the tumor genotype obtained at the time of diagnosis might not accurately represent the genotype of real-time after multiple lines of treatment (19). We wonder whether it is necessary to analyze tumor genotype in real time to predict the clinical activity of apatinib and assess prognosis in patients with mCRC.
Notably, more than 30 patients had the responses of SD in the study, and only four patients were PR (9.8%). Using RECIST version to evaluate the response of therapy, no change was observed in tumor size in some of the patients who had tumor cavitation, particularly in the lungs, or who had reduced central tumor density in the liver based on CT scan. We believe that there was limitation in the use of RESICIST for evaluation of the apatinib response. In some studies, changes have been made to criteria used to evaluate the tumor response of antiangiogenic agents. For instance, Choi was used to evaluate imatinib in GIST (20). In other studies, MASS was used to evaluate sunitinib in renal cancer (21), while mRECIST was used to evaluate sorafenib in hepatocellular carcinoma (22). Therefore, there is an urgent need for appropriate criteria that combines the size and density of tumors, to evaluate antiangiogenic agents such as bevacizumab and apatinib.
In this study, the most frequently observed AEs of apatinib for all grades were hypertension, HFS, proteinuria and leukopenia; which are the most common AEs of antiangiogenic agents (23,24). The grade 3/4 toxicities were HFS, hypertension, leucopenia, proteinuria and neutropenia; and these were mild to moderate in severity. Hoarseness, which has not been reported in other studies (8-10) was observed in six patients. It could be relieved by prednisone, and we estimated that hoarseness was caused by the edema of the vocal cords. There was no statistical correlation between apatinib-related AEs with PFS and OS though it was reported that presence of hypertension, proteinuria, or HFS during the first cycle of apatinib treatment was viable biomarkers of antitumor efficacy in patients with metastatic gastric cancer (25).
Conclusions
The present results suggest that apatinib could be efficiently used for the salvage treatment of mCRC with a manageable side effect profile, which needs to be further confirmed by prospective studies with lager numbers of patients in mCRC.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 81602583).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.04.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Our study obtained the ethics approval by ethics committee of Zhejiang Cancer Hospital, and the number of the approval was IRB-2015-258. Every participant gave informed consent before taking part.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- International Agency for Research on Cancer. GLOBOCAN2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Geneva, Switzerland: World Health Organization, 2013.
- Lemmens V, van Steenbergen L, Janssen-Heijnen M, et al. Trends in colorectal cancer in the south of the Netherlands 1975–2007:rectal cancer survival levels with colon cancer survival. Acta Oncol 2010;49:784-96. [Crossref] [PubMed]
- Van Cutsem E, Borras JM, Castells A, et al. Improving outcomes in colorectal cancer: where do we go from here? Eur J Cancer 2013;49:2476-85. [Crossref] [PubMed]
- Ku G, Tan IB, Yau T, et al. Management of colon cancer: resourcestratified guidelines from the Asian Oncology Summit 2012. Lancet Oncol 2012;13:e470-81. [Crossref] [PubMed]
- Fontanella C, Ongaro E, Bolzonello S, et al. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med 2014;2:123. [PubMed]
- Ding J, Chen X, Dai X, et al. Simultaneous determination of apatinib and its four major metabolites in human plasma using liquid chromatography-tandem mass spectrometry and its application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 2012;895-896:108-15. [Crossref] [PubMed]
- Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011;102:1374-80. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Zhang K, Shi M, Huang C, et al. A phase II, multicenter, placebo-controlled trial of apatinib in patients with advanced non-squamous non-small cell lung(NSCLC) after two previous treatment regimens. J Clin Oncol 2012;30:Abst 7548.
- Hu X, Cao J, Hu W, et al. Multicenter phase II study of Apatinib in non-triple-negative metastatic breast cancer. BMC Cancer 2014;14:820. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. Washington, DC: US Department of Health and Human Services, 2010.
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17. [Crossref] [PubMed]
- Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 2009;360:563-72. [Crossref] [PubMed]
- Grothey A, Van Cutsem E, Sobrero ACORRECT Study Group, et al. Regorafenib monotherapy for previously treatedmetastatic colorectal cancer (CORRECT): an international,multicentre, randomised, placebo-controlled, phase III trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Li J, Qin S, Xu RCONCUR Investigators, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, doubleblind, placebo-controlled, phase III trial. Lancet Oncol 2015;16:619-29. [Crossref] [PubMed]
- Hu X, Zhang J, Xu B, et al. Muticenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer 2014;135:1961-9. [Crossref] [PubMed]
- Riechelmann R, Senna Leite LA, Glasberg J, et al. Regorafenib in antiangiogenic-naive, chemotherapy-refractory advanced colorectal cancer: A phase IIb trial. J Clin Oncol 2018;36:782.
- Tabernero J, Lenz HJ, Siena S, et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: a retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol 2015;16:937-48. [Crossref] [PubMed]
- Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 2007;25:1753-9. [Crossref] [PubMed]
- Smith AD, Shah SN, Rini BI, et al. Morphology, Attenuation, Size, and Structure (MASS) criteria: assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR Am J Roentgenol 2010;194:1470-8. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (Mrecist) assessment for hepaocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Liu L, Wu N, Li J. Novel targeted agents for gastric cancer. J Hematol Oncol 2012;5:31. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJREGARD Trial Investigators, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Liu X, Qin S, Wang Z, et al. Early presence of anti-angiogenesis-related adverse events as a potential biomarker of antitumor efficacy in metastatic gastric cancer patients treated with apatinib: a cohort study. J Hematol Oncol 2017;10:153. [Crossref] [PubMed]