
First year experience with IORT for breast cancer at the Geneva University Hospitals
Introduction
With 5,250 new diagnoses each year, and correspondingly an age standardized rate (ASR) European standard of 111.3 per 100,000 women, breast cancer incidence in Switzerland ranks 15th in Europe (1,2). Within the country, regional disparities have been observed regarding diagnosis and management of the disease (3). In the canton of Geneva, high breast cancer incidence (ASR 128.5, surpassed only by the canton of Vaud’s ASR of 129.8), high proportion of tumors with favorable characteristics, and commensurately low mortality have been ascribed to running programs of mammography screening (3,4). A survey of 1,404 women with operable invasive breast cancer diagnosed in the canton in 2000-2005 found that the majority presented with early stage disease, 50% stage I, 40% stage II (5). Breast conserving surgery was the preponderant surgical procedure. Most women received post-operative radiotherapy. The Geneva University Hospitals (HUG)’s Breast Centre is the public breast cancer unit where two third of these cantonal cases were managed. Radiotherapy has been routinely delivered using fractionation schedules considered safe (6,7), at the cost of extending treatment time over seven weeks. Since many cases in our practice presented with early stage disease, we considered the possibility of reducing the radiation treatment burden by using hypofractionation and partial breast irradiation. The publication of two large series of intra-operative radiotherapy (IORT), by Vaidya et al. (8) and by Veronesi et al. (9), provided good evidence to support the use of IORT. We argued in our national medical journal that it was reasonable to propose IORT to patients with low risk of recurrence (10). IORT was later implemented in our hospital in 2012. The purpose of the present study is to evaluate the characteristics of patients who received IORT and to evaluate early toxicity.
Methods
We retrospectively reviewed the medical records of all women who underwent IORT, from the beginning of its availability at the HUG in February 2012, until January 2013.
Selection of patients
Prior to any therapy, all patients with a newly diagnosed breast cancer referred to the HUG were discussed at a multidisciplinary meeting organized weekly (“concertation d’oncologie sénologie préthérapeutique”, COSP) (5). IORT was proposed to patients after consensus on the a priori eligibility of the patient for breast conserving surgery with IORT, either as exclusive radiation treatment, or as a boost. The HUG eligibility criteria for IORT as exclusive radiation treatment were adapted from the 2009’s recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) regarding accelerated partial breast irradiation (11): age ≥50 years old, histopathology of invasive ductal, mucinous, tubular, medullar or colloid carcinoma, unifocal-unicentric tumor, absence of LVI, absence of extensive in situ component, tumor size ≤30 mm, pathological nodal status pN0 by sentinel node biopsy or pN1mi by axillary dissection, and clear resection margins ≥2 mm. If the criteria were not met, additional whole breast radiotherapy (WBRT) was to be given post-operatively. Patients were excluded from IORT in case of invasive lobular carcinoma (ILC), ductal carcinoma in situ (DCIS), extensive intraductal component (EIC), LVI, or neoadjuvant chemotherapy.
Surgical procedure
IORT was scheduled with the surgery only when the patient provided written informed consent. The patient was admitted to the gynecological surgery ward on the day prior to surgery. Breast harpoon localization by mammography or by ultrasound was done for non-palpable lesions. Lymphoscintigraphy through peri-areolar injection with SPECT/CT was done for the mapping of sentinel nodes. On the day of surgery, the surgical procedure under general anesthesia began with sentinel nodes biopsy. Thereafter, excision of the breast tumor was done, typically through a separate incision except for tumors located in or near the axillary tail of the breast. The resected tissue was inspected by palpation and by radiography. Additional resection of breast tissue was done if it was considered that the tumor or the harpoon was close to a margin. Frozen section pathological examination was done for sentinel nodes, but not for resection margins.
IORT procedure
IORT was done immediately after completion of the tumor excision using the Intrabeam system (Carl Zeiss Surgical GmbH, Oberkochen, Germany) with a spherical applicator. The size of the applicator was chosen according to the size of the resection cavity. The breast tissue surrounding the resection cavity was mobilized in order to appose the tissue on the applicator and was fastened with a purse-string stitch. Skin distance was controlled visually. A moistened gauze was inserted between the skin and the applicator if the applicator’s distance to the hypodermis was estimated to be less than 5 mm. Shielding was not used. A dose of 20 Gy at the surface of the applicator was prescribed. Radiation delivery and anesthesia were monitored outside of the operating room. The applicator was removed after the radiation delivery. The surgery proceeded with an axillary dissection in case of pathological involvement of sentinel nodes. Intravenous antibiotic perfusion was given perioperatively. The IORT procedure, from tumor excision till removal of the applicator, lasted on average one hour. The radiation oncologist jointly participated with the surgeon during the application, and supervised the radiation delivery by a dosimetrist.
External radiotherapy
Definitive pathological results were discussed at a separate multidisciplinary meeting organized weekly for post-surgery cases (“colloque d’oncogynécologie”). For IORT patients, IORT was validated as the sole radiation treatment if the post-operative pathological examination confirmed the eligibility criteria. Otherwise, external WBRT was recommended, with or without regional lymph node irradiation according to pathological lymph node status. External beam radiotherapy was scheduled four and six weeks after surgery-IORT if no adjuvant chemotherapy were given, or four weeks after the last cycle of adjuvant chemotherapy if it was given. External radiotherapy was delivered to the breast at a prescribed dose considered equivalent to 46-50 Gy in 2 Gy fractions through tangential beams. Patients were treated prone if treatment planning showed improved lung sparing with comparable breast coverage (12). In case of supine treatment, right side breasts were treated in free breathing, whereas left side breasts were treated in deep inspiration breath hold under videoscopic control (13). Field-in-field compensation was used as needed to ensure that the 107% isodose volume did not exceed 2 mL (14,15). Radiation was delivered to the ipsilateral axillary supra-clavicular areas if >3 or >20% axillary lymph nodes were involved. Radiation was not delivered to the internal mammary chain.
Data analyses
Patients’ initial data were retrieved from a core database that maintained the list of patients for whom the multidisciplinary COSP proposed IORT. The medical records were abstracted for demographic, clinical, pathological, and treatment characteristics. Toxicity was retrospectively scored from the records at two time points: for all patients, at the first follow-up consultation after surgery-IORT which was nominally scheduled at four weeks post-surgery-IORT, and, for patients who received external beam radiotherapy, at the first follow-up after the end of the radiotherapy which was nominally scheduled at six weeks post-radiotherapy. Toxicity scoring used the Subjective, Objective, Management and Analytic/Late Effects Normal Tissues (SOMA/LENT) system for breast, skin, lung and heart, but without the functional examinations (16-18). The scores were crosschecked with the physicians who examined the patients at different time points. For the purpose of reporting, we graded toxicity as the maximum score observed in any item. We also combined the breast and skin scores retaining only the highest recorded score. Descriptive presentation of the data used cross-tabulations. Significance testing of contingency tables used the Chi-square test. Comparison of means used the Student t-test.
Results
IORT was proposed to 60 patients but was delivered only in 52 cases. The IORT was not done in 8 patients, for preoperative reasons in 3 patients, and was cancelled at the time of operation in the other 5 patients. Preoperatively, 1 patient did not wish to receive any additional information other than the date of her surgery, 1 patient elected to have surgery in another hospital, and 1 patient participated in a preoperative FDG PET/CT trial, the examination found a multifocal tumor. At the time of operation, 1 patient had tumor close to skin, the overlying skin had been resected, the remaining skin was overstretched by approximation of tissues; 1 patient had tumor adherent to pectoralis muscle, there was no specification that the distance of the tumor from the skin and from the muscle should countermand the IORT, but it was considered in this patient that the flat surface at the bottom of the resection cavity would not receive adequate irradiation; 3 patients had extended lumpectomy cavities that did not allow appropriate apposition of breast tissue to the applicators. For the patients receiving IORT, the applicators’ sizes were 2.5 (7.7%), 3 (23%), 3.5 (48%), 4 (9.6%), 4.5 (7.7%), and 5 cm (4%).
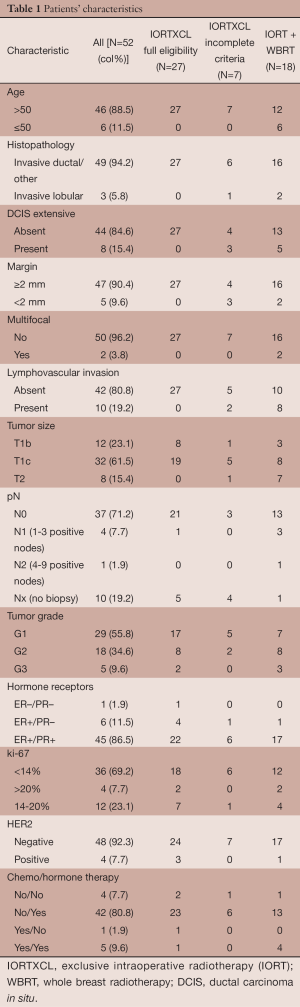
As could be expected from the selection procedure, the 52 women receiving IORT presented a good concordance between pathological characteristics and eligibility for exclusive IORT (Table 1): 88% were older than 50, 94% were invasive ductal carcinoma or other non-lobular types, 90% had resection margins of 2 mm or more, 96% had unifocal breast tumor. One patient had positive resection margin, re-operation found no residual disease, she was considered as fulfilling the margin criteria. Thirty-four (65%) patients had no additional radiotherapy after IORT, of whom 27 fulfilled all eligibility criteria, and 7 did not. Eighteen (35%) received additional WBRT, of whom 1 fulfilled all eligibility criteria for exclusive IORT, and 1 received an additional boost to tumor bed.
Full table
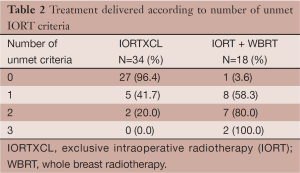
Delivery of WBRT was significantly related to the number of unmet criteria. The proportion of patients receiving WBRT was 3.6%, 58.3%, 80%, and 100% with 0, 1, 2, and 3 unmet criteria, respectively, P<0.0001 (Table 2). There was no case of nodal irradiation. The one patient with four involved axillary nodes had a low lymph node ratio of 16% (4 positive out of 25 examined lymph nodes). WBRT setup was prone in 6 patients, supine free breathing in 9 patients, and supine deep inspiration breath hold in 3 patients. Doses delivered were 15×2.67 Gy (1 patient), 16×2.5 Gy (3 patients), 16×2.66 Gy (6 patients), 20×2.2 Gy (1 patient), 20×2.25 Gy (4 patients), and 21×2.25 Gy (3 patients, 1 with boost 6×2.25 Gy).
Full table
Regarding the seven patients who did not receive WBRT although they did not met full requirement for exclusive IORT, the mean age was 74 years, as compared with mean age of 68 years in the exclusive IORT group with fulfilled criteria, and mean age of 59 years in the IORT with WBRT group, P=0.006. The unmet criteria among these seven patients were: extensive DCIS in 3, resection margin <2 mm in 2, presence of LVI in 1, and combined unmet criteria of ILC combined with resection margin <2 mm in 1 patient. The latter patient was 92 years old. None had multifocal disease, the largest tumor size was 2.4 cm, and axillary lymph node exploration was omitted in 4 of the 7 patients.
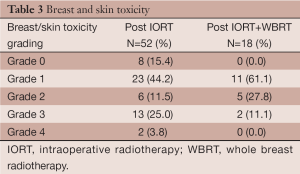
Early toxicities were evaluated in all patients at a median of 27 days (range, 13-70 days) after IORT. There were no heart-related complications. Lung symptoms of cough, dyspnea and chest discomfort scored as Grade 2 were noted in 6 of 52 (11.5%) patients. The symptoms were mild and abated in the following weeks, chest X-rays or CT were not performed. The most frequent breast/skin toxicities were seroma, scored as grade 3 in 13 of 52 (25%) patients (Table 3). Two cases were scored as grade 4: one patient presented wound dehiscence requiring suture; the other patient had immediate bilateral breast augmentation with implants following her tumor resection and IORT, she presented with bilateral hematoma requiring re-operation.
Full table
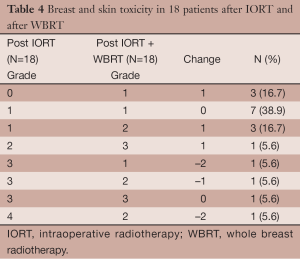
Early post-WBRT evaluation was done at a median of 40 days (range, 19-81 days) after completion of WBRT. There were no heart complication, and only 1 patient presented with mild symptoms of cough and dyspnea. Breast/skin evaluation recorded 2 patients as presenting Grade 3 toxicity, one for persistence of seroma, the other for intense skin dryness. We compared how the grades changed in these 18 patients relatively to their earlier post-IORT evaluation (Table 4). The elapsed time was median 90 days (range, 41-244 days) between the IORT and WBRT evaluations, respectively 86 days among 14 patients without chemotherapy, and 228 days among 4 patients receiving chemotherapy. Grade post-WBRT was increased in 7 patients, comparable in 8 patients, and decreased in 3 patients. Matched pairs analysis showed no significant relationship between the grades at the two time points, P=0.631.
Full table
Prior to submission of the present report, we updated the verification of our patients’ files. As of December 20, 2013, the median follow-up was 370 days (min-max range 27-637 days, interquartile range 227-490 days), there were no recurrences, no grade 4 toxicities.
Discussion
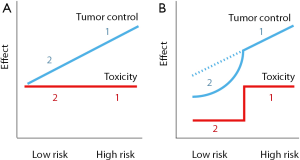
Earlier on like several others we gathered large evidence showing a survival advantage with radiotherapy in breast cancer (19-21). We noted that the proportional reduction of mortality would yield a quite small absolute survival benefit in the case of small node-negative tumors (20). We argued then for partial breast irradiation. The simple rationale is that in low risk tumors, reducing radiotherapy would reduce toxicity. Tumor recurrence would also increase but moderately, the net effect would be a survival gain (Figure 1). The 2010’s publications of the TARGIT-A trial (8) and the Milan’s experience (9) gave confidence to proceed toward implementing IORT in our hospital. The recent update of the TARGIT-A trial demonstrates that overall survival is maintained, and even tend to improve, despite a small increase in breast recurrences (22). A very similar finding was also reported in the ELIOT trial (23).
We opted for a soft X-ray based system on consideration that our selection of patient would be low risk disease that would not require highly penetrating radiation. Indeed our patients’ characteristics presented good prognostic tumor profiles matching well epidemiological surveys of the canton. The number of patients receiving IORT in the present report appears however much lower than what might be expected.
The different WBRT hypofractionation schedules that we used reflect uncertainties during our learning curve. Over the last five years, our department has progressively phased out WBRT of 50 Gy in 25 fractions. Older patients received the Whelan schedule of 42.5 Gy in 16 fractions (24,25) but given four times a week, whereas younger patients received a moderately hypofractionated schedule of 47.25 Gy in 21 fractions given four times a week (6). With IORT delivering 20 Gy in a single fraction, there were concerns that adding WBRT according to these schedules would considerably increase the risk of toxicity. Consequently the prescribed dose was reduced, either by substracting 1 fraction or by reducing the dose per fraction. We took into account that the UK START trial B gave 1 fraction less than the Whelan schedule (26), which suggested a margin for dose reduction of up to 6.25%. Dose reduction was applied to 5 of the first 5 patients then to 3 of the next 6 patients. Thereafter as no unexpected acute toxicities were observed, we applied our usual WBRT schedules except for 1 patient out of 7 who received 40 Gy in 15 fractions.
The IORT dose of 20 Gy in a single shot followed by fractionated WBRT of 50 Gy in 2 Gy fraction-equivalent deserves a particular comment. A single dose of 20 Gy has been considered equivalent to 1.5-2.5 times the same total dose of fractionated external beam radiotherapy (27). That is, a patient given 20 Gy IORT followed by 50 Gy fractionated WBRT would supposedly have received the equivalent of 80-100 Gy, far in excess of the conventional 50 Gy WBRT +16 Gy boost. However, such equivalence approximation does not take into consideration that, unlike intraoperative electron, brachytherapy or external beam radiotherapy which are prescribed on a volume, firstly the Intrabeam dose is prescribed at the surface of the applicator, secondly the dose decreases monotonously with the tissue distance from the applicator, and consequently the radiobiological modelling differs (28). Assuming an applicator size of 4 cm, assuming that the distance from the applicator’s surface where it matters most is 1.0 to 1.5 cm (28), not taking into account the applicator’s handle, the corresponding volume of breast tissue encompassed by the irradiation is 80 to 146 mL, the estimated mean dose to the breast tissue around the applicator is 10.4 Gy (volume within 1 cm) to 7.8 Gy (volume within 1.5 cm). These doses represent 52% to 39% of the nominal value of 20 Gy. Regarding the ipsilateral breast as an organ at risk, Aziz et al. have shown in an anthropormophic phantom dosimetric study that 20 Gy at the surface of a 4 cm applicator delivered to the breast a mean dose of 2.2 Gy (29). By contrast, conventional fractionated boost doses of 16 Gy to tumor bed have been shown to deliver a mean dose of 16 Gy to an average planning target volume (PTV) of 101 +/– 47 mL, and a mean dose to the ipsilateral breast of 7.8-10.5 Gy (30). Accordingly the dose delivered to the breast is reduced four- to five-fold with IORT as compared with conventional boosts. We did not make a direct clinical comparison with conventional external beam radiotherapy. But historically in a group of 50 consecutive patients treated with moderately hypofractionated external beam radiotherapy that we evaluated four years ago, acute G1, G2 and G3 skin toxicity occurred in the boost area of 26%, 60% and 14% patients, respectively (6). That is to say, in line with the dosimetric studies, external beam radiotherapy was associated with slightly more acute toxicity than IORT + WBRT.
The present study has several limitations: small number of patients, retrospective, potential recollection bias, very short follow-up, no patient’s self-assessment. There were no functional lung or heart explorations, neither cosmesis nor quality of life evaluation, there was no formal comparison group. Lack of functional lung-heart explorations might have missed subclinical toxicities (31). Quality of life evaluation would have been valuable to confirm other authors who found less pain, breast and arm symptoms in IORT alone patients as compared with external beam radiotherapy (32). Nevertheless despite the limitations of our study, we believe that sharing one’s experience can be useful, to identify issues and to formulate hypotheses for further researches. One possible issue might be the role of medical imaging. Similarly to Tuschy et al. (33), IORT had to be cancelled in several cases. The happenstance of a patient who underwent a PET/CT raises the question of whether or not it can have a role in the selection of patients. Likewise, we could reflect on the utility of breast MRI prior to IORT (34).
Arguably our use of SOMA/LENT for grading of early toxicities can be considered not optimal. The SOMA/LENT is intended for evaluation of late toxicities. However we plan to evaluate our patients in a few years. We felt using the same scoring system throughout in order to compare the toxicity grades over time would facilitate that follow-up.
Although the current follow-up is short, we found that Intrabeam IORT is a safe technique that did not prevent further radiotherapy. Our experience is in line with other authors who have reported low rates of late toxicities with longer follow-up when using IORT as boost (35,36). Compared to the TARGIT-A trial in which the rate of additional WBRT as per treatment was 15.2% (22), our 35% rate of WBRT was considerably larger. This might be related to different selection criteria. We noted that age and the number of unmet criteria were significant factors in the delivery of additional WBRT. The importance of age as a potential issue will have to be debated in the selection of patients (37). Other issues are the role of hormone receptors, which we did not take into consideration in the current guidelines, and the role of LVI. We considered LVI as an exclusion criterion for IORT. However, LVI or other high risk prognostic factors could in fact be major indication for IORT in order to deliver radiation at the time of surgery. This could be a challenging hypothesis that might be tested in future researches.
Conclusions
In our early experience, we found that Intrabeam IORT was a safe procedure. Toxicity of IORT was moderate. It was not significantly increased in patients receiving WBRT. The technique deserves to be made more readily available to our patients.
Acknowledgments
IORT as a reality at the HUG owes to late Georges Vlastos, founder and head of the onco-senology unit.
Heartfelt thanks to Olena Gorobets of the Cité Hospitalière de Mangot-Vulcin for her precious editorial assistance, to Maryam Ackermann-Zare of the HUG for her great help with the follow-up of patients, and to Edite Richard for her careful maintenance of the patients database.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Frederik Wenz and Elena Sperk) for the series “Intraoperative Radiotherapy” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Virginie Nepote was partly funded by private funds for IORT raised by Georges Vlastos and Vincent Vinh-Hung and managed by the HUG. The authors declare no conflict of interest.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval was waived informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bouchardy C, Lutz JM, Kühni C. Cancer in Switzerland: situation and development from 1983 to 2007. Neuchatel: FSO, 2011.
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [PubMed]
- Ess S, Savidan A, Frick H, et al. Geographic variation in breast cancer care in Switzerland. Cancer Epidemiol 2010;34:116-21. [PubMed]
- NICER. National statistics on cancer incidence. Interactive statistics map. Zurich, Switzerland: National Institute for Cancer Epidemiology and Registration, 2013.
- Taban F, Rapiti E, Fioretta G, et al. Breast cancer management and outcome according to surgeon’s affiliation: a population-based comparison adjusted for patient’s selection bias. Ann Oncol 2013;24:116-25. [PubMed]
- Vock J, Peguret N, Balmer-Majno S, et al. 254 Four times weekly adjuvant breast radiotherapy with a moderately intensified boost to the tumour bed - feasibility and acute toxicity. EJC Suppl 2010;8:132-3.
- Van de Steene J, Soete G, Storme G. Adjuvant radiotherapy for breast cancer significantly improves overall survival: the missing link. Radiother Oncol 2000;55:263-72. [PubMed]
- Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 2010;376:91-102. [PubMed]
- Veronesi U, Orecchia R, Luini A, et al. Intraoperative radiotherapy during breast conserving surgery: a study on 1,822 cases treated with electrons. Breast Cancer Res Treat 2010;124:141-51. [PubMed]
- Vlastos G, Monnier S, Vinh-Hung V. Innovations in locoregional treatments of breast cancer. Rev Med Suisse 2010;6:2016-2018-23. [PubMed]
- Polgár C, Van Limbergen E, Pötter R, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother Oncol 2010;94:264-73. [PubMed]
- Fargier O, Grozema F, Laouiti M, et al. DVH comparison of whole breast radiotherapy (WBRT) in prone and supine position. Abs OC-0349. Radiother Oncol 2013;106:S136-7.
- Vinh-Hung V, Grozema F, Lee YE, et al. Single Institution Review of Setup Errors with Deep-Inspiration Breath Hold (DIBH) for Left Sided Breast RT. Abstract 41. SASRO, Geneva, Mar 31 - Apr 2, 2011.
- Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol 2008;26:2085-92. [PubMed]
- Barnett GC, Wilkinson J, Moody AM, et al. A randomised controlled trial of forward-planned radiotherapy (IMRT) for early breast cancer: baseline characteristics and dosimetry results. Radiother Oncol 2009;92:34-41. [PubMed]
- . LENT SOMA tables. Radiother Oncol 1995;35:17-60. [PubMed]
- Rubin P, Constine LS 3rd, Fajardo LFEORTC Late Effects Working Group, et al. Overview of late effects normal tissues (LENT) scoring system. Radiother Oncol 1995;35:9-10. [PubMed]
- Pavy JJ, Denekamp J, Letschert JEORTC Late Effects Working Group, et al. Late effects toxicity scoring: the SOMA scale. Radiother Oncol 1995;35:11-5. [PubMed]
- Whelan TJ, Julian J, Wright J, et al. Does locoregional radiation therapy improve survival in breast cancer? A meta-analysis. J Clin Oncol 2000;18:1220-9. [PubMed]
- Vinh-Hung V, Verschraegen C. Breast-conserving surgery with or without radiotherapy: pooled-analysis for risks of ipsilateral breast tumor recurrence and mortality. J Natl Cancer Inst 2004;96:115-21. [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [PubMed]
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. [PubMed]
- Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013;14:1269-77. [PubMed]
- Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst 2002;94:1143-50. [PubMed]
- Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010;362:513-20. [PubMed]
- START Trialists’ Group. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 2008;371:1098-107. [PubMed]
- Calvo FA, Meirino RM, Orecchia R. Intraoperative radiation therapy first part: rationale and techniques. Crit Rev Oncol Hematol 2006;59:106-15. [PubMed]
- Herskind C, Griebel J, Kraus-Tiefenbacher U, et al. Sphere of equivalence--a novel target volume concept for intraoperative radiotherapy using low-energy X rays. Int J Radiat Oncol Biol Phys 2008;72:1575-81. [PubMed]
- Aziz MH, Schneider F, Clausen S, et al. Can the risk of secondary cancer induction after breast conserving therapy be reduced using intraoperative radiotherapy (IORT) with low-energy x-rays? Radiat Oncol 2011;6:174. [PubMed]
- Toscas JI, Linero D, Rubio I, et al. Boosting the tumor bed from deep-seated tumors in early-stage breast cancer: a planning study between electron, photon, and proton beams. Radiother Oncol 2010;96:192-8. [PubMed]
- Verbanck S, Hanon S, Schuermans D, et al. Small airways function in breast cancer patients before and after radiotherapy. Breast Cancer Res Treat 2012;135:857-65. [PubMed]
- Welzel G, Boch A, Sperk E, et al. Radiation-related quality of life parameters after targeted intraoperative radiotherapy versus whole breast radiotherapy in patients with breast cancer: results from the randomized phase III trial TARGIT-A. Radiat Oncol 2013;8:9. [PubMed]
- Tuschy B, Berlit S, Nasterlack C, et al. Intraoperative radiotherapy of early breast cancer using low-kilovoltage x-rays-reasons for omission of planned intraoperative irradiation. Breast J 2013;19:325-8. [PubMed]
- Horst KC, Fero KE, Ikeda DM, et al. Defining an optimal role for breast magnetic resonance imaging when evaluating patients otherwise eligible for accelerated partial breast irradiation. Radiother Oncol 2013;108:220-5. [PubMed]
- Wenz F, Welzel G, Blank E, et al. Intraoperative radiotherapy as a boost during breast-conserving surgery using low-kilovoltage X-rays: the first 5 years of experience with a novel approach. Int J Radiat Oncol Biol Phys 2010;77:1309-14. [PubMed]
- Sperk E, Welzel G, Keller A, et al. Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: results from the randomized phase III trial TARGIT A. Breast Cancer Res Treat 2012;135:253-60. [PubMed]
- Azria D, Lemanski C. Intraoperative radiotherapy for breast cancer. Lancet 2014;383:578-81. [PubMed]


