
Anthracycline cardiotoxicity: new actors on the stage
Anthracyclines, including doxorubicin that was discovered in Italy over a half century ago, are widely used chemotherapeutics for the treatment of many human cancers (1). Shortly after their introduction, the cardiovascular toxicity has been reported (2), but despite decades of research, the pathogenesis of cardiotoxicity is still incompletely understood.
Contrary to the common perception, the dimension of anthracycline cardiotoxicity is by no means small. A recent analysis, conducted on patients treated with anthracyclines with a follow-up of 9 years, reported a rate of 17.9% and 6.3% of subclinical and overt cardiotoxicity, respectively (3). The use of alternative echocardiographic parameters such as myocardial strain, documented even higher incidence of anthracycline-induced cardiac dysfunction, rating about 30% in adult survivors of childhood cancer (4). Given the growing population of cancer survivors exposed to the treatment as children or adults, cardiotoxicity has attracted more attention in the new discipline of cardio-oncology. This recent initiative aims at promoting research of mechanisms driving cardiotoxicity and bringing uniformity to the guidelines regarding diagnosis, management and monitoring (5).
In a recently published Circulation Research paper, Gupta and colleagues reported a previously unrecognized phenomenon involved in the pathogenesis of anthracycline cardiotoxicity (6). By global transcriptional profiling approach, the researchers identified doxorubicin-induced alterations in the levels of several cardiac RNA-binding proteins (RBPs). In particular, the downregulation of Quaking isoform 5 (Qki5) was observed. RBPs are known to control the function of coding and noncoding RNAs. Regulatory function of RNA is a relatively late-studied aspect of cell biology, given that more than 98% of the transcriptional output of the human genome is noncoding RNA (7,8). Qki was shown to regulate the formation of circular RNAs and its pro-survival properties on cardiomyocytes has been previously reported in ischemic myocardium (9). By using in vivo and in vitro overexpression of Qki5, Gupta and colleagues documented a protective role of this protein against doxorubicin-induced cell death and cardiac dysfunction. The effects of Qki5 were dependent on the dose and subcellular localization. Several circular RNAs, controlled by overexpression of Qki5, were also downregulated in response to doxorubicin. Interestingly, inhibition of Ttn-derived circular RNA increased the susceptibility of cardiomyocytes to doxorubicin. Thus, an extensive transcriptomic approach by Gupta and colleagues identified Qki5 as an important mediator of doxorubicin cardiotoxicity and highlighted the role circular RNAs in this pathology (Figure 1).
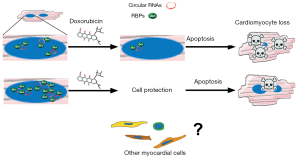
The doxorubicin-induced cardiotoxicity involves multiple molecular mechanisms and, up to date, the formulation of a unified model of pathogenesis has not clearly been defined. To properly maintain hemodynamic function, the human heart requires an adequate supply of oxidative energy in each one of billion cardiomyocytes. In this regard, reactive oxygen species (ROS) accumulation and involvement of mitochondria as a subcellular target of doxorubicin have been studied in the context of a cardiomyocyte as a cell type particularly rich in mitochondria. It is generally recognized that oxidative stress leads to the activation of necrotic and apoptotic pathways causing cardiomyocyte loss (10).
A series of strategies have been used in attempt to reduce harmful effects that anthracyclines have on myocardial structure and function. The first approach to diminish the incidence of cardiotoxicity is to use a therapeutic dose lower than 450 mg/m2. However, lowering the cumulative dose of doxorubicin causes a significant reduction of on-treatment events, but has no impact on late-onset complications, indicating that no dose of doxorubicin that can be considered safe (11). For example, hearts of children exposed to a dose of doxorubicin below 100 mg/m2, show structural abnormalities in 30% of survivors several years after cancer diagnosis (12). Pharmacokinetics-based method consists of changing administration schedule by replacing bolus with slow infusion and switching from conventional to liposomal formulations, although inconclusive results and elevated costs limit their use. Additionally, less cardiotoxic anthracycline derivatives, such as epirubicin or idarubicin, are not routinely used in current practice (13).
Dexrazoxane is the only approved cardioprotective agent against anthracycline cardiotoxicity, with a documented efficacy in both children and adult patients (14,15). As an iron chelating agent, it interferes with iron-dependent redox reactions thereby decreasing ROS production and tissue damage. Recently, as additional mode of action, dexrazoxane was shown to deplete DNA topoisomerase IIβ, thus preventing anthracycline-induced DNA double strand breaks (16). The clinical use of dexrazoxane was limited by regulatory agencies following a concern, subsequently disproven, regarding the potential risk of a second malignancy in paediatric patients (17). Other compounds with anti-oxidant properties, such as probucol, vitamin E, L-carnitine, coenzyme Q, glutathione and N-acetylcysteine were tested in experimental and clinical settings with inconclusive findings (18). Although supported by a strong rationale, the antioxidant therapy targeting redox signalling in anthracycline cardiomyopathy has failed to give a significant impact to protect form cardiotoxic risk. Now it is clear that subcellular compartmentalization of ROS and ROS-mediated signalling is central for both cardiovascular physiology and response to stress (19). This should pave the way for the development of new intervention strategies targeting antioxidants to specific compartments, interfering with a specific ROS-dependent subcellular pathway, thus favouring beneficial effects over the location-unspecific action.
The treatment, as for patients with heart failure, includes a combination of β-blockers, ACE-inhibitors, angiotensin receptor blockers (ARBs), diuretics, nitrates and hydralazine (20). In particular, ACE-inhibitors and β-blockers have showed a significant cardiac protection in patients under anthracycline treatment. Recently, ESC Committee for Practice Guidelines has recommended the use of ACE-inhibitors (or ARBs) and β-blockers in patients with heart failure or asymptomatic cardiac dysfunction (21). Overall, available cardioprotective measures has not solved the clinical problem. Therefore, the work of Gupta and colleagues regarding the possibility to target RBPs in the cardio-oncologic setting offers an alternative scenario that is worth deepening.
Similar to the myriad of studies aiming to explore cellular and molecular phenomena, the study of Gupta and colleagues focused on cardiomyocytes that account for less than one third of the total number of cells within the heart. However, other cell types such as cardiac fibroblasts, endothelial cells, undifferentiated cells and vascular smooth muscle cells are also present and are involved in the homeostasis of the heart. Because these entities dynamically interact in response to changes in homeostatic and pathological stimuli, the structural and functional relationship between different cellular components cannot be dismissed (22-24). In fact, Gupta and colleagues detected the expression of Qki5, 6 and 7 in cardiac fibroblasts and endothelial cells raising a possibility that the role of Qki family may extend beyond cardiomyocyte compartment.
In summary, the novel work of Gupta and colleagues reveals previously unrecognized aspects of cardiotoxicity. Adding a new level of complexity to myocardial pathophysiology, this study not only significantly contributes to new specialized field of research but also calls for follow up research aiming at extending our understanding of the role of noncoding RNA biology in other cardiovascular diseases.
Acknowledgments
Funding: This work was supported by PON 03 PE_00060_8; PON 03 PE_00060_7; POR Campania FESR 2007-2013 “Ockey-Oncology and Cardiology Key Target”.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Chunlin Ou (Cancer Research Institute of Central South University, Changsha, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.04.24). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grein A, Spalla C, Di Marco A, et al. Descrizione e classificazione di un attinomicete (streptomyces peucetius sp. nova) produttore di una sostanza ad attività antitumorale: la daunomicina. Giorn Microbiol 1963;11:109-18.
- Bonadonna G, Monfardini S, De Lena M, et al. Clinical evaluation of adriamycin, a new antitumour antibiotic. Br Med J 1969;3:503-6. [Crossref] [PubMed]
- Lotrionte M, Biondi-Zoccai G, Abbate A, et al. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol 2013;112:1980-4. [Crossref] [PubMed]
- Armstrong GT, Joshi VM, Ness KK, et al. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results rrom the St. Jude lifetime cohort study. J Am Coll Cardiol 2015;65:2511-22. [Crossref] [PubMed]
- Ewer M, Gianni L, Pane F, et al. Report on the international colloquium on cardio-oncology (Rome, 12-14 March 2014). Ecancermedicalscience 2014;8:433. [PubMed]
- Gupta SK, Garg A, Bär C, et al. Quaking inhibits doxorubicin-mediated cardiotoxicity through regulation of cardiac circular RNA expression. Circ Res 2018;122:246-54. [Crossref] [PubMed]
- Bonetti A, Carninci P. From bench to bedside: the long journey of long non-coding RNAs. Curr Opin Syst Biol 2017;3:119-24. [Crossref]
- Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014;157:77-94. [Crossref] [PubMed]
- Guo W, Jiang T, Lian C, et al. QKI deficiency promotes FoxO1 mediated nitrosative stress and endoplasmic reticulum stress contributing to increased vulnerability to ischemic injury in diabetic heart. J Mol Cell Cardiol 2014;75:131-40. [Crossref] [PubMed]
- Minotti G, Menna P, Salvatorelli E, et al. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 2004;56:185-229. [Crossref] [PubMed]
- Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 2005;23:2629-36. [Crossref] [PubMed]
- Leger K, Slone T, Lemler M, et al. Subclinical cardiotoxicity in childhood cancer survivors exposed to very low dose anthracycline therapy. Pediatr Blood Cancer 2015;62:123-7. [Crossref] [PubMed]
- Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol 2014;64:938-45. [Crossref] [PubMed]
- Lipshultz SE, Scully RE, Lipsitz SR, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol 2010;11:950-61. [Crossref] [PubMed]
- Speyer JL, Green MD, Zeleniuch-Jacquotte A, et al. ICRF-187 permits longer treatment with doxorubicin in women with breast cancer. J Clin Oncol 1992;10:117-27. [Crossref] [PubMed]
- Lyu YL, Kerrigan JE, Lin CP, et al. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res 2007;67:8839-46. [Crossref] [PubMed]
- Asselin BL, Devidas M, Chen L, et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma: a report of the Children’s Oncology Group Randomized Trial Pediatric Oncology Group 9404. J Clin Oncol 2016;34:854-62. [Crossref] [PubMed]
- Ladas EJ, Jacobson JS, Kennedy DD, et al. Antioxidants and cancer therapy: a systematic review. J Clin Oncol 2004;22:517-28. [Crossref] [PubMed]
- Brown DI, Griendling KK. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ Res 2015;116:531-49. [Crossref] [PubMed]
- Gianni L, Herman EH, Lipshultz SE, et al. Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol 2008;26:3777-84. [Crossref] [PubMed]
- Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768-801. [Crossref] [PubMed]
- Cappetta D, Rossi F, Piegari E, et al. Doxorubicin targets multiple players: A new view of an old problem. Pharmacol Res 2018;127:4-14. [Crossref] [PubMed]
- Piegari E, Russo R, Cappetta D, et al. MicroRNA-34a regulates doxorubicin-induced cardiotoxicity in rat. Oncotarget 2016;7:62312-26. [Crossref] [PubMed]
- Prezioso L, Tanzi S, Galaverna F, et al. Cancer treatment-induced cardiotoxicity: a cardiac stem cell disease? Cardiovasc Hematol Agents Med Chem 2010;8:55-75. [Crossref] [PubMed]


