Hypertension and risk of cholangiocarcinoma: a systematic review and meta-analysis
Introduction
Cholangiocarcinoma (CCC), initially described by Durand Fardel in 1840, is a malignant cancer originated from the epithelium of bile duct (1,2). CCC represents the second most common primary hepatic carcinoma, accounting for 3% of all gastrointestinal malignancies as well as 10–25% of liver malignancies (3,4). Moreover, in recent decades, the incidence of CCC is still rising. Intriguingly, the epidemiology of intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC) are different, with an increasing incidence of the former, but a decreasing incidence of the latter in certain regions worldwide, including the UK and the USA (5). In the past 20 years, the incidence of ICC has been elevated by 165%, while that of ECC has been declined by 14% in the US (6). In addition, the prognosis of CCC is very poor. The relative survival rates of 1, 3 and 5 years have been reported to be 25%, 9.7% and 6.8%, respectively, almost any change in recent decades (7,8). And the cause of CCC is not yet fully understood, with only several confirmed risk factors of CCC, including gallstones, primary sclerosing cholangitis, parasitic infections as well as bile-duct cysts (9). Recently, additional indicators affecting CCC risks have been determined by multiple meta-analyses, including cirrhosis, alcohol consumption, smoking, and diabetes mellitus (10-14). In recent years, increasing attention has been paid to hypertension for its relationship with endometrial cancer and breast cancer (15,16). Moreover, the association between hypertension and CCC has been documented in case-control studies. However, its association is controversial. To this end, this systematic review with meta-analysis enrolling published observational researches was carried out to obtain a better understanding of the correlation of hypertension with the risk of CCC.
Methods
This study was performed in accordance with PRISMA statement (17) as well as MOOSE guidelines (18).
Data sources and search strategy
Web of Science, EMBASE as well as PubMed databases were thoroughly searched using the following keywords: (“blood Pressure” OR “hypertension” OR “metabolic syndrome” OR “risk factor”) and (“biliary tract cancer” OR “bile duct cancer” OR “biliary tract neoplasms” OR “cholangiocarcinoma”). Language of article or date of publication was not restricted.
Inclusion criteria
The inclusion criteria were listed in the following: study design (cohort or case control); hypertension as an exposure factor while CCC/biliary tract cancer/bile duct cancer as an outcome; and accessible odds ratio (OR)/risk ratio (RR) values as well as corresponding 95% CIs or adequate data for calculation. In the case of the same outcomes shown by two researches, study with larger sample would be chosen.
Data abstraction and quality assessment
Two investigators (J Lin, J Long) independently collected the demanded data from all enrolled studies in a standard manner. The following data were obtained from each study: study design, sources of controls, first author’s name, country of origin, year of publication, number of subjects, adjusted confounding variables, duration of follow-up as well as OR/RR values and 95% CIs.
The Newcastle-Ottawa Scale (NOS) (19) was utilized to determine study quality. Quality categories were assigned in line with the scores of each study. The maximal score was 9 points. To be specific, NOS scores of <4, 4–6, and 7–9 suggested low, moderate, and high quality, respectively (20). Discrepancies were resolved by consensus.
Statistical analysis
The OR/RR values and 95% CIs were employed to determine CCC risk in hypertensive patients. Random effects model was used to determine the association of hypertension with CCC risk, which was proposed by DerSimonian and Laird (21).
The I2 statistic was utilized to determine the heterogeneity between studies, where I2 value of 25%, 50%, and 75% implicated low, moderate, and high heterogeneity, respectively (22). A P value <0.1 implicated obvious heterogeneity. Meta-regression was used to evaluate the extent to which heterogeneity between the study results was correlated with geographical locations, and confounders adjusted for (smoking status, alcohol use, gallstones). Funnel plot and Begg’s (23) and Egger’s (24) tests were employed to investigate publication bias, where funnel plot asymmetry as well as a P value <0.05 implicated publication bias.
Subgroup analyses were carried out according to subtype of cancer, gallstones, alcohol consumption, geography smoking, and whether liver fluke infestation after adjustment. Sensitivity analysis was carried out to investigate whether the outcomes were stable by sequential omission of each study. Moreover, sensitivity analysis was also conducted by changing the pooling model (fixed-effects model or random-effects model) to eliminate studies with NOS score <7.
STATA version 12.0 was utilized for statistical analysis.
Results
Study selection and study characteristics
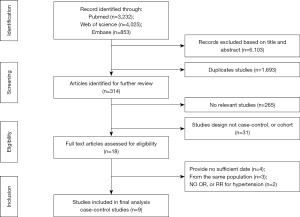
The selection procedure was shown in Figure 1. As a result, 8,110 articles were initially obtained (3,232, 4,025, and 853 from PubMed, EMBASE, and Web of Science, respectively), 1,693 of which were duplicates. After eliminating further 6,103 studies on the basis of title and abstract, nine studies were excluded after evaluating the full texts, because they did not meet the inclusion criteria: four studies provided insufficient information (25-28), three articles reported on the same population (29-31), and two studies did not provide OR, or RR for hypertension or sufficient information to calculate these variables (32,33). Thus, nine eligible observational studies were enrolled in this meta-analysis (34-42).
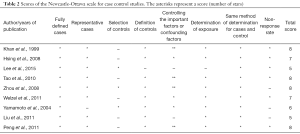
The major features of the enrolled researches were summarized in Table 1, all of which were case-control studies, ranging from 1980 to 2013. Five, two, one and one studies were conducted in China, the USA, Korea and Japan, respectively. Specifically, in this study, 2,016 patients with CCC and 199,812 healthy controls were enrolled to probe into the effect of hypertension on the risk of CCC. The NOS scores of nine studies varied from 5 to 8, with six high-quality studies as well as three moderate ones (Table 2).

Full table
Full table
Association of cholecystectomy with CCC risk
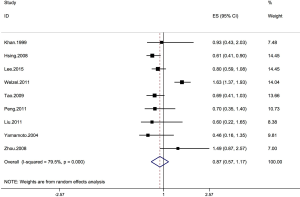
Nine case-control studies were enrolled to probe into the correlation of hypertension with the risk of CCC (34-42). One study demonstrated that significantly higher risk of CCC was observed in hypertensive subjects compared to those of healthy controls (35). Another research showed that hypertension was correlated with increased risk of CCC (37). However, the above correlation was not found in the remaining researches (34,36,38-42). The pooled estimate of hypertension influence was insignificant (OR, 0.87; 95% CI, 0.57–1.17), with significantly heterogeneous studies (I2=79.5%; P=0.000) (Figure 2). In addition, this correlation was also detected in ECC (OR, 0.74; 95% CI, 0.42–1.37, I2=0%), instead of ICC (OR, 1.07; 95% CI, 0.43–1.71, I2=83.5%) (Table 3).

Full table
Subgroup and sensitivity analyses
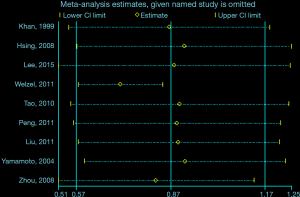
The outcomes of subgroup analysis as well as sensitivity analysis were shown in Table 3. In terms of sensitivity analysis, despite elimination of researches with NOS sources under 7, the correlation of hypertension with the risk of CCC was still stable (Table 3). In addition, the overall outcomes for the association of hypertension with CCC was stable despite changed pooling model (fixed-effects model: OR, 0.88; 95% CI, 0.76–1.01; random-effects model: OR, 0.87; 95% CI, 0.57–1.17) (Table 3). Moreover, another approach of sensitivity analysis was conducted to explore this heterogeneity, where pooled ORs were calculated by sequential omission of one study every time. The most substantial variation of pooled ORs in sensitivity analysis and the I2 was observed when Welzel et al. [2011] (37) was eliminated. Without Welzel et al. [2011] (37), the pooled OR for the effect of hypertension on CCC was 0.58 (95% CI, 0.65–0.82). The I2 values was 37.4% (P=0.13), demonstrating remarkably declined heterogeneity in comparison to when Welzel et al. [2011] (37) was enrolled (Figure 3). Meta-regression analysis was also performed to probe into the potential source of heterogeneity. However, meta-regression models did not indicate that the geographical locations (P=0.910), and confounders adjusted for smoking status (P=0.738), alcohol use (P=0.819), gallstones (P=0.904) were a source of heterogeneity.
Publication bias
The funnel plot failed to show substantial asymmetry, neither did Begg’s or Egger’s tests detect any substantial publication bias (P>0.05) (Figure 4).

Discussion
To our knowledge, this meta-analysis is the first to probe into the correlation of hypertension and CCC risk. Nine studies were selected to assess the influence of hypertension on CCC risk, which revealed that hypertension is not significantly correlated with the risk of CCC (OR, 0.87; 95% CI, 0.57–1.17), among significantly heterogeneous studies. In the separation analysis of ICC and ECC, the correlation between hypertension and elevated risk of ECC (OR, 0.74; 95% CI, 0.42–1.37) or ICC (OR, 1.07; 95% CI, 0.43–1.71) was not significant, either.
Our study harbors certain advantages. To begin with, it is the first large-scale meta-analysis (2,016 patients with CCC and 199,812 healthy volunteers) to determine the influence of hypertension on CCC risk. Hence, our results might provide novel insight into the above-described relationship, which is likely to be significant to CCC research. Moreover, subgroup as well as sensitivity analyses were carried out to figure out the influencing factors of the outcomes, which enhances the reliability of our results. Thirdly, a comprehensive search of Web of Science, EMBASE as well as PubMed databases was conducted to select potential studies, aiming at the investigation of the correlation of hypertension with the risk of CCC. Last but not least, the majority of enrolled studies were of high quality. Together, the above factors contribute to the convincingness of this meta-analysis.
There exist certain limitations in the present study. To begin with, all enrolled researches were case-control studies, possibly leading to recall as well as selection biases. In addition, there was significant heterogeneity among studies due to diverse study designs as well as inconsistency of demographic characteristics. Although we were not always able to ascertain the source of heterogeneity, we have performed several sensitivity and meta-regression to address this issue. Secondly, we only assessed CCC risk in hypertensive patients compared to healthy controls. None of the selected studies provided the stages or grades of hypertension and risk of CCC; hence, we were unable to carry out a dose-response analysis to more accurately evaluate the correlation between these variables. Thirdly, in this study, we only assessed a potential correlation, vulnerable to confounding bias. The established risk factors for CCC include parasitic infections, bile-duct cysts, hepatolithiasis as well as primary sclerosing cholangitis (9), which, however, were only adjusted in a few studies. Moreover, our findings are vulnerable to diagnostic bias. Hypertensive subjects are prone to receive physical examination, which may contribute to the early detection of CCC.
Conclusions
In summary, available evidence from observational studies indicates that hypertension has no association with elevated risk of CCC. However, more prospective studies as well as basic research are warranted to verify the association of hypertension with CCC risk.
Acknowledgments
Funding: This work was funded by International Science and Technology Cooperation Projects (No. 2015DFA30650 and 2016YFE0107100), The Capital Special Research Project for the clinical application (No. Z151100004015170).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology 2004;66:167-79. [Crossref] [PubMed]
- Vijgen S, Terris B, Rubbia-Brandt L. Pathology of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2017;6:22-34. [Crossref] [PubMed]
- Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis 1994;14:109-14. [Crossref] [PubMed]
- Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004;24:115-25. [Crossref] [PubMed]
- Taylor-Robinson SD, Toledano MB, Arora S, et al. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968-1998. Gut 2001;48:816-20. [Crossref] [PubMed]
- Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472-7. [Crossref] [PubMed]
- Lepage C, Cottet V, Chauvenet M, et al. Trends in the incidence and management of biliary tract cancer: a French population-based study. J Hepatol 2011;54:306-10. [Crossref] [PubMed]
- Pawlik TM. Intrahepatic cholangiocarcinoma: from diagnosis to treatment. Hepatobiliary Surg Nutr 2017;6:1. [Crossref] [PubMed]
- Khan SA, Thomas HC, Davidson BR, et al. Cholangiocarcinoma. Lancet 2005;366:1303-14. [Crossref] [PubMed]
- Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol 2012;57:69-76. [Crossref] [PubMed]
- Zhou Y, Zhao Y, Li B, et al. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: evidence from a meta-analysis. BMC Cancer 2012;12:289. [Crossref] [PubMed]
- Wang Z, Sheng YY, Dong QZ, et al. Hepatitis B virus and hepatitis C virus play different prognostic roles in intrahepatic cholangiocarcinoma: A meta-analysis. World J Gastroenterol 2016;22:3038-51. [Crossref] [PubMed]
- Li M, Li J, Li P, et al. Hepatitis B virus infection increases the risk of cholangiocarcinoma: a meta-analysis and systematic review. J Gastroenterol Hepatol 2012;27:1561-8. [Crossref] [PubMed]
- Jing W, Jin G, Zhou X, et al. Diabetes mellitus and increased risk of cholangiocarcinoma: a meta-analysis. Eur J Cancer Prev 2012;21:24-31. [Crossref] [PubMed]
- Han H, Guo W, Shi W, et al. Hypertension and breast cancer risk: a systematic review and meta-analysis. Sci Rep 2017;7:44877. [Crossref] [PubMed]
- Aune D, Sen A, Vatten LJ. Hypertension and the risk of endometrial cancer: a systematic review and meta-analysis of case-control and cohort studies. Sci Rep 2017;7:44808. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 2009;3:e123-30. [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Zhang YP, Li WQ, Sun YL, et al. Systematic review with meta-analysis: coffee consumption and the risk of gallstone disease. Aliment Pharmacol Ther 2015;42:637-48. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Ioannou GN. Cholelithiasis, cholecystectomy, and liver disease. Am J Gastroenterol 2010;105:1364-73. [Crossref] [PubMed]
- Urbach DR, Swanstrom LL, Khajanchee YS, et al. Incidence of cancer of the pancreas, extrahepatic bile duct and ampulla of Vater in the United States, before and after the introduction of laparoscopic cholecystectomy. Am J Surg 2001;181:526-8. [Crossref] [PubMed]
- Huang YJ, Wu AT, Chiou HY, et al. Interactive role of diabetes mellitus and female sex in the risk of cholangiocarcinoma: A population-based nested case-control study. Oncotarget 2017;8:6642-51. [PubMed]
- Songserm N, Promthet S, Sithithaworn P, et al. Risk factors for cholangiocarcinoma in high-risk area of Thailand: role of lifestyle, diet and methylenetetrahydrofolate reductase polymorphisms. Cancer Epidemiol 2012;36:e89-94. [Crossref] [PubMed]
- Hsing AW, Gao YT, Han TQ, et al. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer 2007;97:1577-82. [Crossref] [PubMed]
- Liu E, Sakoda LC, Gao YT, et al. Aspirin use and risk of biliary tract cancer: a population-based study in Shanghai, China. Cancer Epidemiol Biomarkers Prev 2005;14:1315-8. [Crossref] [PubMed]
- Shebl FM, Andreotti G, Rashid A, et al. Diabetes in relation to biliary tract cancer and stones: a population-based study in Shanghai, China. Br J Cancer 2010;103:115-9. [Crossref] [PubMed]
- Zhang GW, Lin JH, Qian JP, et al. Identification of risk and prognostic factors for patients with clonorchiasis-associated intrahepatic cholangiocarcinoma. Ann Surg Oncol 2014;21:3628-37. [Crossref] [PubMed]
- Petrick JL, Freedman ND, Graubard BI, et al. Coffee Consumption and Risk of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma by Sex: The Liver Cancer Pooling Project. Cancer Epidemiol Biomarkers Prev 2015;24:1398-406. [Crossref] [PubMed]
- Khan ZR, Neugut AI, Ahsan H, et al. Risk factors for biliary tract cancers. Am J Gastroenterol 1999;94:149-52. [Crossref] [PubMed]
- Hsing AW, Zhang M, Rashid A, et al. Hepatitis B and C virus infection and the risk of biliary tract cancer: a population-based study in China. Int J Cancer 2008;122:1849-53. [Crossref] [PubMed]
- Lee BS, Park EC, Park SW, et al. Hepatitis B virus infection, diabetes mellitus, and their synergism for cholangiocarcinoma development: a case-control study in Korea. World J Gastroenterol 2015;21:502-10. [Crossref] [PubMed]
- Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology 2011;54:463-71. [Crossref] [PubMed]
- Tao LY, He XD, Qu Q, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a case-control study in China. Liver Int 2010;30:215-21. [Crossref] [PubMed]
- Peng NF, Li LQ, Qin X, et al. Evaluation of risk factors and clinicopathologic features for intrahepatic cholangiocarcinoma in Southern China: a possible role of hepatitis B virus. Ann Surg Oncol 2011;18:1258-66. [Crossref] [PubMed]
- Yamamoto S, Kubo S, Hai S, et al. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci 2004;95:592-5. [Crossref] [PubMed]
- Zhou YM, Yin ZF, Yang JM, et al. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol 2008;14:632-5. [Crossref] [PubMed]
- Liu ZY, Zhou YM, Shi LH, et al. Risk factors of intrahepatic cholangiocarcinoma in patients with hepatolithiasis: a case-control study. Hepatobiliary Pancreat Dis Int 2011;10:626-31. [Crossref] [PubMed]