
Specific skin changes induced by chemotherapy
Introduction
The latest statistics showed that the year of 2015 witnessed 4,292,000 new cancer patients arise and 2,814,000 patients would eventually succumb to the disease. Cancer has been the leading cause of death in China and is one of the key issues threatening public health due to its increasing incidence and mortality (1). Apart from being used to treat cancer, chemotherapy also plays a pivotal role in treating blood disorders and some autoimmune diseases. Despite the considerable development of targeted therapy which substantially prolongs the survival of patients, chemotherapy in oncology is nonetheless essential, especially in adjuvant and neoadjuvant therapy (2-4). However, one of the common adverse reactions caused by chemotherapy is skin toxicity though it does not attract sufficient attention as it does not lead to fatal outcomes. These adverse reactions generally display complex and diverse features on the skin of immunocompromised patients, and have a negative impact on these cancer patients, both mentally and physically. The physical presentation of these symptom could reduce the patients’ treatment compliance and living quality (5). Hereby, four case reports regarding the side effects of chemotherapy on skin are presented and the relevant articles are reviewed and discussed.
Case presentation
Case 1
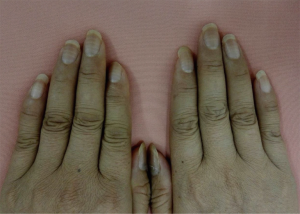
A 43-year-old female presented to our department with one-month ulceration of right lower gingiva in March of year 2015. She had a resection of neoplasm from right lower gingiva on March 30th 2015 and the pathological diagnosis was extra-nodal NK-T cell lymphoma. The positron emission tomography-computed tomography (PET-CT) has revealed diffusive lymphoma with lung infiltration. She has received etoposide, doxorubicin, vincristine, cyclophosphamide and prednisone (EPOCH) chemotherapy for 8 cycles from April 14th to October 18th in 2015. The treatment evaluation after 4 cycles of EPOCH was partial response (PR, the evaluations were done according to the RECIST guidelines, namely Response Evaluation Criteria in Solid Tumors) whereas after 8 cycles was complete response (CR). After completion of 6 cycles of chemotherapy, Muehrcke’s lines emerged on all of the patient’s nails (Figure 1). When she returned to our department for follow-up after 3 months from the last chemotherapy, the Muehrcke’s lines had disappeared.
Case 2
Female patient, 44 years old, had undergone left modified radical mastectomy with left axillary sentinel lymph node biopsy successfully on June 16th 2015 because of a left breast mass. Grossly, the excised mass was approximately 2.2 cm × 1.8 cm × 1.7 cm. The histopathological analysis of the mass showed that it was invasive ductal carcinoma of the left breast (stage III) and is strongly positive for human epidermal growth factor receptor-2 (HER-2) but negative for estrogen receptor (ER) or progesterone receptor (PgR) with high expression of Ki-67 (80%). A few vessels with cancer embolism were observed while none of the nine resected nodes was found with carcinoma metastasis. She had 4 cycles of epirubicin and cyclophosphamide (EC) followed by 4 cycles of paclitaxel liposome and trastuzumab (TH) as adjuvant chemotherapy from June 26th to August 31th 2015. She showed good tolerance during the EC followed by TH chemotherapy after the operation. Muehrcke’s lines were also observed on her nails after 4 cycles of chemotherapy (Figure 2). Three months after chemotherapy, Muehrcke’s lines vanished without intervention.

Case 3
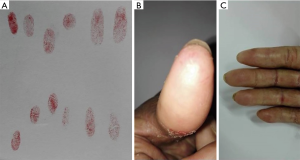
A 64-year-old female came to our department with a mass on her left breast. After systemic examination, she underwent a modified radical mastectomy with left axillary sentinel lymph node biopsy of the left breast was done on June 25th 2010. The microscopic observation revealed mucinous carcinoma of the breast, 5.0 cm × 5.0 cm × 3.5 cm in size, with immunohistochemistry of ER(++), negative expression for both PgR and HER-2 and low expression of Ki-67 (10%) and no tumor metastasis found on the nine resected nodes. Six cycles of the adjuvant chemotherapy with cyclophosphamide and docetaxel (DC) were then performed. During the follow-up on October 12th for reexamination, the CT detected multiple carcinomas in hilar and mediastinal lymph nodes of both lungs. She then received chemotherapy of gemcitabine, endocrine therapy of sequentially anastrozole, exemestane and toremifene from October 2010 to April 2014, all of which were failed eventually. The CT in the follow-up on May 22nd 2013 showed new bone and bilateral adrenal glands metastasis. Forty cycles of capecitabine in total were administered from May 2013 to August 2015. All of her treatment evaluations of the subsequent follow-ups for the capecitabine regime were stable disease (SD). Notably, she experienced slight finger stiffness and loss of fingerprints in most of her fingers in August 2015 when returned for review (Figure 3). She was advised to increase vegetables and fruits intake to obtain sufficient natural vitamins instead of administrating more pills. In the following 5 months, she continued the treatment of capecitabine until disease progression. When she returned to our clinic after capecitabine was terminated for 2 months, physical examination showed that the symptom of finger stiffness has improved while her fingerprints were recovered.
Case 4
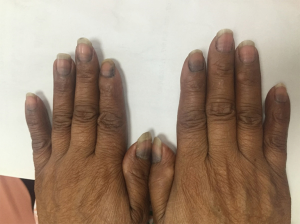
A 57-year-old female presented to our department in October 2015. After a needle biopsy of the right breast mass and relevant radiological examinations, she was clinically diagnosed with right breast invasive ductal carcinoma (T2N0M0) according to the TNM classification, with the molecular classification of luminal B and HER-2 positive. From October 13th to December 15th 2015, she received EC for 4 cycles as neoadjuvant therapy. The right breast mass of the 4th patient has shrunk from 2.6 cm × 1.5 cm to 2.4 cm × 1.3 cm in gross measurement according to ultrasound imaging. The post-treatment evaluation for the neoadjuvant chemotherapy of EC was SD. During treatment, the patient was found to have multiple black pigmentation throughout her body, of which there were band-like or diffusive black pigmentation on her nails (Figure 4). We tried but failed to contact the patient during the follow-up.
Discussion
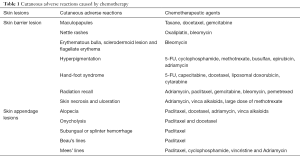
The case reports above are unique and rare skin changes caused by chemotherapy, which are rarely reported in oncology cases. Alkylating agent, including vinblastine and vincristine, one of the widely-used anti-tumor drugs, is likely to induce multiple side effects on skin and mucosa, with diverse and complex symptoms including alopecia, paronychia, dry skin, pigmentation, and skin rashes (5). The occurrence of these symptom not only increase pain to the patients, but also tend to decrease their compliance for the treatment. Until now, the presentation of specific skin changes induced by chemotherapy has been side-tracked and their relevant systemic reviews are rare. Herein we present a thorough review concerning two major classes of common skin toxicity caused by chemotherapy on the basis of the reported cases and previous literatures. We reviewed pertinent publications retrieved by a selective search in PubMed from 1950 to 2017 regarding skin toxicity due to chemotherapy with the inclusion of the following key terms (including “skin”, “cutaneous”, “toxicity”, “reaction” or “change”). After careful scrutinization, we identified common skin toxicity induced by chemotherapy. Table 1 lists the adverse dermatological effects caused by chemotherapy and we summarize the incidence of the adverse dermatological effects caused by chemotherapy on Table 2.
Full table
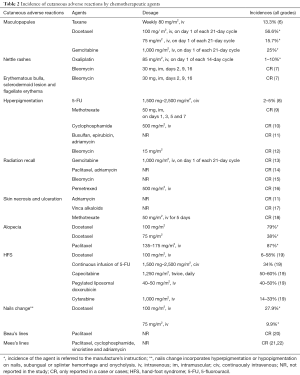
Full table
Skin barrier lesions
Skin rash
Skin rash is a common manifestation of skin allergy on chemotherapy. For example, taxanes are widely utilized in the treatment for solid tumor. Both paclitaxel and docetaxel can lead to maculopapules on faces, limbs and butts, and might cause secondary infection of pustular rash (23,24). Gemcitabine, an anti-metabolism chemical drug, also causes skin rashes associated with allergy and itching (25). Other anti-cancer drugs such as oxaliplatin and bleomycin may present patient with either typical nettle-rashes or non-specific ones. One retrospective study shows that over 15% of the patients experience hypersensitivity during the treatment with oxaliplatin (26). The analysis points out that being a male and an increase in eosinophils of the peripheral blood are two indicators to be susceptible to oxaliplatin-related hypersensitivity reactions. Bleomycin, used mainly for Hodgkin’s lymphoma, germinoma and malignant pleural effusion, results in various skin rashes such as eczematous change, erythematous bulla, sclerodermoid lesion and infrequent flagellate erythema (7).
Hyperpigmentation
Hyperpigmentation refers to the abnormal increase of melanin, which can be subdivided into diffuse and local hyperpigmentation according to its region. The former has been reported to appear after the prescription of 5-fluorouracil (5-FU), cyclophosphamide, methotrexate and busulfan (9,10), and the latter occurred in patients who received adriamycin, 5-FU, cyclophosphamide and busulfan, which may have local hyperpigmentation in their mouths. Bleomycin may cause pigmentation on the body induced by exogenous sources of pressure, as seen our case presentation 4 patient whose nails was presented with diffuse or band-like pigmentation after 4 cycles of epirubicin (12). The occurrence of the band-like shape might be the discontinuation of adriamycin drugs and the recovery in the growth of the nail matrix, thus changing the color of nails (19).
Hand-foot syndrome (HFS)
HFS frequently happens on compression zones, such as palms and plantars. The clinical manifestations are numbness, paresthesia, skin swelling, erythema, desquamation and severe pain. This is especially common for patients who receive 5-FU or capecitabine. After oral administration of capecitabine, more than half of the patients suffered from HFS (19). A retrospective study indicates that for the gastroenteric cancer patients, the occurrence of side effects after the usage of the combination of capecitabine with oxaliplatin was similar to that of pumping 5-FU intravenously (13). An analysis based on a randomized trial shows that HFS induced by capecitabine might be the independent predictor in prolonging the overall survival (OS) of colon cancer patients (27). Other chemotherapeutic drugs that have been reported to induce HFS include docetaxel, liposomal doxorubicin, cytarabine among others (24,28). Incidence of HFS may be reduced by local cooling, using urea ointment and vitamin B6 (19). Nevertheless, these methods are still short of clinical evidences. The 4th patient presented received capecitabine for 40 cycles, and grade II HFS developed with the loss of fingerprints. Likewise, Chavarri-Guerra et al. in 2015 reported a breast cancer patient who was denied by a bank to authorize a financial transaction due to her loss of fingerprints. The author explained that the phenomena of fading fingerprints may be an outcome of HFS induced by capecitabine (29).
Radiation recall
Radiation recall refers to inflammation led by chemotherapeutic drugs in previously irradiated tissues, such as: skin, mucosa, lung, esophagus, intestine and heart with unknown mechanisms. Different from radiation damage, radiation recall develops several months to years after termination of radiotherapy. It has been reported that patients who have received adriamycin and paclitaxel bear more risk of radiation recall. Besides that, gemcitabine, bleomycin and pemetrexed were reported as well (14-16,30).
Skin appendage lesions
Alopecia
About 65% of the patients who receive chemotherapy may suffer from alopecia (31). Most chemotherapeutic drugs like paclitaxel, adriamycin, vinca alkaloids can lead to alopecia, which is hair loss and even short hair loss, including eyebrow, eyelashes and armpit hair, etc. However, hair will regenerate within 3–6 months from the discontinuation of chemotherapeutic drugs generally, while only very few patients will develop permanent hair loss (32,33). It might be useful to prevent hair loss by wearing ice cap or using tourniquet during chemotherapy to constrict local vessels and reduce the intake of chemotherapeutic drugs. However, the side effects of this method is the possibility of metastasis to the scalp (34,35).
Nail change
Both paclitaxel and docetaxel can lead to onycholysis. Other changes caused by paclitaxel, including subungual hemorrhage, splinter hemorrhage, Beau’s lines, and Mees’ lines (20,21). Beau’s lines are a series of traversing depressed lines through the nail, which indicates the inhibition or pause of nail matrix mitosis (36). The depth of the depressions reflects the duration and severity of these lesions. Cyclophosphamide, Vincristine and Adriamycin may lead to Mees’ lines-white lines paralleling lunula, as an outcome of impaired keratinization of the nail matrix (22). Both of the lines will move forward to the tip as nail grows.
Similarly, Muehrcke’s lines are white lines lie parallel to lunula and extend across the nail. In fact, these are the signs of loss of pigmentation, as a consequence of nail bed rather than the influence to the nail itself. Therefore, the lines will not alter as the nails grow. It was reported that the formation of Muehrcke’s lines may be attributed to hypoalbuminemia. Various diseases accompanied by hypoalbuminemia, such as: glomerular diseases, infections and so on, might also cause Muehrcke’s lines, while replenishing albumins can possibly makes the lines fade (20,37). As seen in our cases, the first patient’s serum albumin has decreased to as low as 35 g/L, while the second patient did not have hypoalbuminemia during her treatment. It still warrants further study to explore the association between Muehrcke’s lines and hypoalbuminemia. The methods to identify each of the lines are listed below (Table 3).
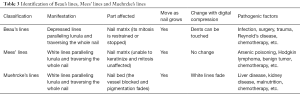
Full table
Other skin changes caused by chemotherapy were dry skin and desquamation. Strong irritating chemotherapeutic drugs like adriamycin and vinca alkaloids can lead to skin necrosis and ulceration if exuding out of vessels. Large dose of methotrexate would possibly result in vesiculation and ulceration of the skin even if rescued by calcium folinate (38).
Prospective
Skin changes induced by chemotherapy are extremely common and are often neglected. Not only should clinicians focus on the therapeutic results, more attentions should be paid to the skin toxicity of chemotherapy. It is crucial to prevent and treat skin changes as early as possible during the treatment, for it is highly related to the quality of patient’s life and compliance to the treatment. Studies show that from the beginning of chemotherapy, skin care managements like proper skin cleaning and application of certain dermatological products were deemed to be effective in reducing the incidence of skin side effects (21). For most of the skin changes, perhaps physical therapy treatment like topical cooling weigh more advantages in treatment than utilizing certain chemical products. Further studies are warranted to investigate the ideal methods to prevent and cope with skin changes induced by chemotherapy.
Acknowledgments
Funding: This work was supported by a grant from the Key Laboratory of Malignant Tumor Molecular Mechanism and Translational Medicine of Guangzhou Bureau of Science and Information Technology {grant No. [2013]163}, a grant from the Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of Guangdong Higher Education Institutes (grant No. KLB09001), grants from the National Natural Science Foundation of China (grant No. 81372819, 81572596, U1601223), a grant from the Guangdong Science and Technology Department (grant No. 2015B050501004), funding from the Guangzhou Science and Technology Bureau (No. 2014J4100170, 201704020131), and a grant from the Guangdong Natural Science Foundation (grant No. 2017A030313828).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.17). The authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Zhang G, Wang H, Zhang M, et al. Current Status and Development of Traditional Chemotherapy in Non-small Cell Lung Cancer under the Background of Targeted Therapy. Zhongguo Fei Ai Za Zhi 2015;18:587-91. [PubMed]
- Ryan CW, Desai J. The past, present, and future of cytotoxic chemotherapy and pathway-directed targeted agents for soft tissue sarcoma. Am Soc Clin Oncol Educ Book 2013; [Crossref] [PubMed]
- Schneeweiss A, Ruckhäberle E, Huober J. Chemotherapy for Metastatic Breast Cancer - An Anachronism in the Era of Personalised and Targeted Oncological Therapy? Geburtshilfe Frauenheilkd 2015;75:574-83. [Crossref] [PubMed]
- Fabbrocini G, Cameli N, Romano MC, et al. Chemotherapy and skin reactions. J Exp Clin Cancer Res 2012;31:50. [Crossref] [PubMed]
- Grau JJ, Mverger C. Weekly paclitaxel for platin-resistant stage IV head and neck cancer patients. Acta Otolaryngol 2009;129:1294-9. [Crossref] [PubMed]
- Lee HY, Lim KH, Ryu Y, et al. Bleomycin-induced flagellate erythema: A case report and review of the literature. Oncol Lett 2014;8:933-5. [Crossref] [PubMed]
- Falkson G, Schulz EJ. Skin changes in patients treated with 5FU. Br J Dermatol 1962;74:229-36. [Crossref] [PubMed]
- Dasari P, Sagili H. Life-threatening complications following multidose methotrexate for medical management of ectopic pregnancy. BMJ Case Rep 2012;2012. doi:
10.1136/bcr-03-2012-6023 . - Dave S, Thappa DM. Peculiar pattern of nail pigmentation following cyclophosphamide therapy. Dermatol Online J 2003;9:14. [PubMed]
- Cakir B, Sucak G, Haznedar R. Longitudinal pigmented nail bands during hydroxyurea therapy. Int J Dermatol 1997;36:236-7. [Crossref] [PubMed]
- Yaris N, Cakir M, Kalyoncu M, et al. Bleomycin induced hyperpigmentation with yolk sac tumor. Indian J Pediatr 2007;74:505-6. [Crossref] [PubMed]
- Delavan JA, Chino JP, Vinson EN. Gemcitabine-induced radiation recall myositis. Skeletal Radiol 2015;44:451-5. [Crossref] [PubMed]
- Masri SC, Misselt AJ, Dudek A, et al. Radiation recall reaction causing cardiotoxicity. J Cardiovasc Magn Reson 2014;16:25. [Crossref] [PubMed]
- Stelzer KJ, Griffin TW, Koh WJ. Radiation recall skin toxicity with bleomycin in a patient with Kaposi sarcoma related to acquired immune deficiency syndrome. Cancer 1993;71:1322-5. [Crossref] [PubMed]
- Boesmans S, Decoster L, Schallier D. Pemetrexed-induced radiation recall dermatitis of the breast. Anticancer Res 2014;34:1179-82. [PubMed]
- Koralewski P. Treatment of skin changes after extravenous administration of adriamycin. Wiad Lek 1987;40:1689. [PubMed]
- Fırat C, Erbatur S, Aytekin AH. Management of extravasation injuries: a retrospective study. J Plast Surg Hand Surg 2013;47:60-5. [Crossref] [PubMed]
- Miller KK, Gorcey L, McLellan BN. Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol 2014;71:787-94. [Crossref] [PubMed]
- Muehrcke RC. The Finger-nails in Chronic Hypoalbuminaemia. BMJ 1956;1:1327-8. [Crossref] [PubMed]
- Haley AC, Calahan C, Gandhi M, et al. Skin care management in cancer patients: an evaluation of quality of life and tolerability. Support Care Cancer 2011;19:545-54. [Crossref] [PubMed]
- Piraccini BM, Iorizzo M. Drug reactions affecting the nail unit: diagnosis and management. Dermatol Clin 2007;25:215-21. vii. [Crossref] [PubMed]
- Kate S, Gulia S, Gupta S. Severe skin toxicity due to weekly paclitaxel administration. Indian J Med Paediatr Oncol 2015;36:62. [Crossref] [PubMed]
- Poi MJ, Berger M, Lustberg M, et al. Docetaxel-induced skin toxicities in breast cancer patients subsequent to paclitaxel shortage: a case series and literature review. Support Care Cancer 2013;21:2679-86. [Crossref] [PubMed]
- Earle CC, Stewart DJ, Cormier Y, et al. A phase I study of gemcitabine/cisplatin/etoposide in the treatment of small-cell lung cancer. Lung Cancer 1998;22:235-41. [Crossref] [PubMed]
- Okayama T, Ishikawa T, Sugatani K, et al. Hypersensitivity Reactions to Oxaliplatin: Identifying the Risk Factors and Judging the Efficacy of a Desensitization Protocol. Clin Ther 2015;37:1259-69. [Crossref] [PubMed]
- Hofheinz RD, Heinemann V, von Weikersthal LF, et al. Capecitabine-associated hand-foot-skin reaction is an independent clinical predictor of improved survival in patients with colorectal cancer. Br J Cancer 2012;107:1678-83. [Crossref] [PubMed]
- Nowara E, Huszno J. Skin toxicity after palliative chemotherapy containing pegylated liposomal doxorubicin for ovarian cancer patients. Ann Palliat Med 2013;2:71-5. [PubMed]
- Chavarri-Guerra Y, Soto-Perez-de-Celis E. Loss of Fingerprints. N Engl J Med 2015;372:e22 [Crossref] [PubMed]
- Duncker-Rohr V, Freund U, Momm F. Radiation recall dermatitis after docetaxel chemotherapy. Strahlenther Onkol 2014;190:491-3. [Crossref] [PubMed]
- Dua P, Heiland MF, Kracen AC, et al. Cancer-related hair loss: a selective review of the alopecia research literature. Psychooncology 2017;26:438-43. [Crossref] [PubMed]
- Palamaras I, Misciali C, Vincenzi C, et al. Permanent chemotherapy-induced alopecia: A review. J Am Acad Dermatol 2011;64:604-6. [Crossref] [PubMed]
- Dorr VJ. A practitioner's guide to cancer-related alopecia. Semin Oncol 1998;25:562-70. [PubMed]
- Dmytriw AA, Morzycki W, Green PJ. Prevention of alopecia in medical and interventional chemotherapy patients. J Cutan Med Surg 2015;19:11-6. [Crossref] [PubMed]
- Chon SY, Champion RW, Geddes ER, et al. Chemotherapy-induced alopecia. J Am Acad Dermatol 2012;67:e37-47. [Crossref] [PubMed]
- Ben-Dayan D, Mittelman M, Floru S, et al. Transverse Nail Ridgings (Beau’s Lines) Induced by Chemotherapy. Acta Haematol 1994;91:89-90. [Crossref] [PubMed]
- Sharma V, Kumar V. Muehrcke lines. CMAJ 2013;185:E239 [Crossref] [PubMed]
- Scheinfeld N. Three cases of toxic skin eruptions associated with methotrexate and a compilation of methotrexate-induced skin eruptions. Dermatol Online J 2006;12:15. [PubMed]


