
It is feasible to perform laparoscopic pancreaticoduodenectomy for patients with prior abdominal operation
Introduction
Intra-abdominal surgeries may induce scarring and bowel adhesions to the abdominal wall. Approximately 30–50% of severe complications associated with laparoscopic surgery may occur during surgical access, and a major risk factor is previous upper abdominal operation (PUAO) (1). A history of intra-abdominal surgery has been reported to be a relative contraindication for laparoscopic surgery (2). However, rapid developments in technological innovations, improvements in surgical skills, and accumulated operative experience have expanded the indications for laparoscopic surgeries. Some surgeons began to perform laparoscopic surgeries in patients who previously underwent abdominal operations (2-6). Performing laparoscopic surgery in these patients is safe, although this procedure is associated with an increased risk of operative complications, a high conversion rate, and a prolonged operating time (5,6).
Pancreatectomy is a kind of highly specialized operation, and laparoscopic pancreaticoduodenectomy (LPD) is one of the most challenging laparoscopic pancreatic operations (7). Adhesion caused by previous abdominal surgery causes complications during surgical access and considerably increases the difficulty in performing dissections and reconstructions during LPD. To our knowledge, this is the first study to comparatively assess the feasibility of LPD in patients who underwent PUAO.
Methods
We performed 282 LPD cases, including 42 cases with PUAO (group 1), from November 2010 to May 2017. A cohort of 84 patients was matched in terms of age, gender, body mass index, American Society of Anesthesiology, and histopathologic diagnosis [42 cases without PUAO (group 2)]. All of the surgeries were performed by a single surgeon, and data were collected and analyzed retrospectively in terms of demographic characteristics, intraoperative variables (operation time, estimated blood loss, and conversion rate), postoperative variables (postoperative hospital stay and complications). Written consent was obtained from the patients enrolled in this study, and this work was permitted by the Ethics Committee of Sichuan University.
Operative procedures
All of the patients were given general anesthesia and placed in a supine position with legs apart and a 20° head-up tilt. Generally, five trocars were used. A 12 mm trocar was placed at the lower umbilicus for a 10 mm 30° camera. Two 12 mm main manipulating trocars were placed at the bilateral medioclavicular line 1–2 cm above the umbilical level. Two 5 mm trocars were placed at the bilateral anterior axillary line subcostally. At the beginning of all operations, a full laparoscopic abdominal exploration was performed to exclude liver metastases and abdominal dissemination. A window in the gastrocolic ligament was created and enlarged to reveal the entire pancreas by using a harmonic scalpel (Ethicon Endo-surgery, Cincinnati, OH, USA). An extended mobilization of the hepatic flexure of the colon was carried out, and an extended Kocher maneuver was performed to expose the inferior vena cava and the aorta. The right gastroepiploic vein and artery were then dissected and transected. The superior mesenteric vein (SMV) was identified at the lower edge of the pancreas, and a tunnel was created between the posterior wall of the pancreas and the SMV/portal vein. After the vessels of the smaller curvature of the stomach were dissected, the distal one-third of the stomach or the duodenum [in the case of laparoscopic pylorus-preserving pancreaticoduodenectomy (LPPPD)] was transected by using a 60 mm endoscopic linear cutting stapler (Ethicon Endo-Surgery). Cholecystectomy was performed, and the common bile duct at the confluence of the hepatic ducts and the pancreas neck were transected with an ultrasonic scalpel. The jejunum was transected approximately 15 cm to Treitz’s ligament by utilizing an endoscopic linear cutting stapler. The mesentery of the uncinate process was completely dissected, and standard lymphadenectomy was carried out. The specimen was placed in a retrieval bag and retrieved from a 4 cm incision around the umbilicus. The proximal jejunal stump was delivered through a window in the transverse mesocolon. The digestive tract was reconstructed in the following order: pancreaticojejunostomy, hepaticojejunostomy, and gastrojejunostomy. Pancreaticojejunostomy was conducted with an end-to-side and duct-to-mucosa running suture with an internal intent. An end-to-side hepaticojejunostomy was carried out with a 4-0 monofilament absorbable running suture. Side-to-side gastrojejunostomy was conducted with an endoscopic linear cutting stapler. In LPPPD cases, the end-to-side duodenojejunostomy was performed with 3-0 monofilament absorbable suture lines. Three prophylactic drainages were placed near pancreatic anastomosis and hepaticojejunostomy.
We adopted the policy of attempting laparoscopic surgery for all of the patients with PUAOs. The initial access to the abdominal cavity was around the umbilicus in all of the cases either by a blind or open technique. For patients with previous supraumbilical intraperitoneal operations, the first trocar was placed at least 3 cm away from the previous incision to avoid organ injury. The distributions of the trocars were slightly adjusted on the basis of the adhesions between the intestinal tracts and the abdominal wall. Once the surgeon reached the peritoneal cavity, adhesiolysis was adequately carried out to expose the operative field and establish the accurate definition of the anatomy. The remaining operative procedures were the same as those for patients without PUAOs.
Definitions
Operative time was defined as the time from the first skin incision to the skin closure. Overall morbidity was described as any complication associated with the operation within 30 days of surgery. Pancreatic fistula was graded A–C as defined by the International Study Group on Pancreatic Fistula (8). Delayed gastric emptying after pancreatic surgery was defined by the International Study Group of Pancreatic Surgery (9). Length of hospital stay was calculated from the day of surgery through the day of discharge.
Statistical analysis
Numerical data were expressed as mean ± standard deviation. Statistical analyses were performed using SPSS 16.0 for Windows. Differences between variables were compared using Student’s t-test, chi-square test, or Fisher’s exact test. Data were considered significant at P<0.05.
Results
Table 1 shows the demographic characteristics of the patients included in this study. The mean age of the patients in the group 1 was 61.2±6.3 years. The most frequent indication for surgery was ampullary adenocarcinoma, followed by cholangiocarcinoma and pancreatic ductal adenocarcinoma. The most common type of PUAO was laparoscopic cholecystectomy (14 cases) followed by open cholecystectomy (11 cases) and common bile duct exploration (7 cases). Three patients with previous abdominal surgery suffered from the adhesive ileus and underwent open enterolisis. Three patients who suffered from gastric perforation received an open repair of gastric perforation. One patient with refractory duodenal ulcer was treated with open subtotal gastrectomy. Two patients were subjected to cholangiojejunostomy for choledochal cyst and common bile duct stones, respectively. One patient with abdominal abscess was performed with open drainage of abscess. Overall, 16 patients underwent previous laparoscopic surgery, and 26 patients received previous open surgery. Furthermore, 34 patients underwent previous abdominal surgery once, 6 patients received prior abdominal surgeries twice, and 2 patients underwent prior abdominal surgery thrice. The patients in the two groups were well matched in terms of age, gender, body mass index, tumor size, tumor location, and histopathologic diagnosis.
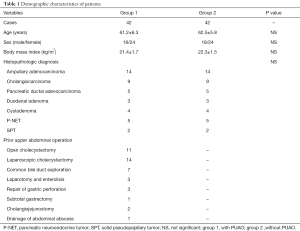
Full table
The operative outcomes and postoperative details are shown in Table 2. One patient in the group 1 was converted to hand-assisted surgery because of extensive adhesion between the patient’s small intestines caused by abdominal abscess. The mean time for adhesiolysis in the group 1 was 16 min, but the total operative time was comparable between the two groups (392±41 vs. 385±33 min, P=0.217). The estimated blood loss was also comparable between the two groups (147±32 vs. 162±43 mL, P=0.142). No patient in both groups required blood transfusion. No statistical difference was observed between the two groups in terms of mean time to the first passage of flatus and postoperative hospital stay. Moreover, no 30-day mortality was reported in both groups. We used an open technique to insert the first trocar in the high-risk patients who were suspected to suffer from extensive adhesion. No complication occurred during the first trocar placement in our series. One patient was injured in the small intestine during adhesiolysis, and the injury needed repair. This patient was discharged on the ninth postoperative day uneventfully. Seven patients in group 1 suffered from pancreatic fistula. Of these patients, six cases of grade A and one case of grade B pancreatic fistula were included. One patient suffered from biliary fistula and three patients experienced delayed gastric emptying. These patients were cured with conservative treatment. One patient suffered from abdominal fluid collection, which required percutaneous drainage. The overall complications were comparable between the two groups.
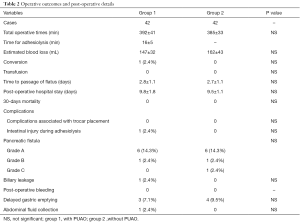
Full table
Discussion
Laparoscopic surgery is associated with less invasiveness, short recovery time, reduced morbidity, and enhanced cosmetic results. This procedure has been widely accepted as an alternative to conventional open surgery in many gastrointestinal fields, including left-sided pancreatic resections. In 1994, Gagner et al. (10) reported that LPD is associated with long operative time, lack of apparent advantages, and advanced laparoscopic skills. With the development of operative instruments and the accumulation of operative experience, many studies have reported that LPD is safe and feasible compared to open surgery.
PUAO is associated with difficulty in inserting the first trocar and obtaining adequate exposure of the operating field. Moreover, PUAO increases the risk of injury of organs adherent to the abdominal wall during trocar insertion and adhesiolysis. Few retrospective studies have explored the safety and feasibility of laparoscopic surgery in patients with PUAO. Diez et al. (11) performed 411 cases of laparoscopic cholecystectomy in patients with previous infraumbilical intraperitoneal surgery. No morbidity caused by trocars or adhesiolysis was reported in their study. They concluded that previous abdominal operations are not contraindications for laparoscopic cholecystectomy. Karayiannakis et al. (2) also performed 473 cases of laparoscopic cholecystectomy to patients with previous abdominal surgery and concluded that previous upper abdominal surgery is associated with the need for adhesiolysis, increased open conversion rate, and prolonged operating time. Tsunoda et al. (12) performed 22 cases of laparoscopic gastrectomy to patients who underwent upper abdominal surgery and concluded that this procedure is safe and feasible and that PUAO should not be regarded as a contraindication for laparoscopic gastrectomy. In comparison with these laparoscopic surgeries, LPD is associated with considerably complex organ dissections, necessity for rigorous adhesiolysis for explicit anatomy, and complicated alimentary tract reconstructions, resulting in a prolonged operative time and increased operative morbidity. However, the safety and feasibility of LPD for patients who have undergone PUAO remain unclear. In this study, the average duration for adhesiolysis was 16 min, but the average operative time was comparable between the two groups. No significant difference was observed between the two groups in terms of estimated blood loss, transfusion rate, conversion rate, and complications.
Of note, the first trocar should be placed safely. Intestinal injury could happen during the placement of the first trocar. We found that blindly placing the Veress needle and the first trocar 3 cm away from the abdominal scar could be safe. However, we implemented an open technique to insert the first trocar in high-risk patients who were suspected to suffer from extensive adhesion. No complications associated with the insertion of the Veress needle or any of the trocars were reported in our series. Once the surgeon reached the peritoneal cavity, adhesiolysis was performed carefully by using an ultrasonic dissector or electrocautery scissors. Technically, adhesiolysis can be performed easily after the creation of a pneumoperitoneum, which elevates the abdominal wall to provide an enhanced dissection plane for laparoscopy (12).
With the lack of tactile sensation, further attention should be given to the aberrant hepatic artery. We performed computed tomography angiography for each patient to identify vessel variation. For patients who underwent a previous surgery associated with the hepatoduodenal ligament, we should avoid causing hepatic artery injury during adhesiolysis, especially in patients with aberrant hepatic artery. We conducted electrocautery to reveal the anatomy of hepatic artery and its branches. With three anastomoses, rigorous adhesiolysis is required for the explicit anatomy of the small intestine. In our series, we performed two cases of LPD with previous Roux-en-Y cholangiojejunostomy. We dismantled the previous anastomosis and dissected the jejunum between bilioenteric anastomosis and jejunal anastomosis. However, identifying the right anatomy was technically challenging. In such cases, Treitz’s ligament is a hallmark used to find previous jejunal anastomosis and distal jejunum. One patient was required to convert to hand-assisted surgery because of extensive adhesion between the small intestines caused by abdominal abscess.
Our study had several important limitations. First, our study was retrospective. Potential selection bias in matched pair analysis could not be easily ruled out. Second, our study reported short-term outcomes, and long-term outcomes are unavailable.
Conclusions
LPD can be safely and feasibly applied to patients with PUAO. PUAO should not be regarded as a contraindication for LPD. We recommend that a policy to attempt laparoscopic surgery should be adopted for all patients, including those who underwent previous complicated upper abdominal operations.
Acknowledgments
Funding: This study was funding by National Institutes of Health of China (W2017ZWS07).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.27). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written consent was obtained from the patients enrolled in this study, and this work was permitted by the Ethics Committee of Sichuan University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chi I, Feldblum PJ, Balogh SA. Previous abdominal surgery as a risk factor in interval laparoscopic sterilization. Am J Obstet Gynecol 1983;145:841-6. [Crossref] [PubMed]
- Karayiannakis AJ, Polychronidis A, Perente S, et al. Laparoscopic cholecystectomy in patients with previous upper or lower abdominal surgery. Surg Endosc 2004;18:97-101. [Crossref] [PubMed]
- Rafii A, Camatte S, Lelievre L, et al. Previous abdominal surgery and closed entry for gynaecological laparoscopy: a prospective study. BJOG 2005;112:100-2. [Crossref] [PubMed]
- Morris L, Ituarte P, Zarnegar R, et al. Laparoscopic adrenalectomy after prior abdominal surgery. World J Surg 2008;32:897-903. [Crossref] [PubMed]
- Goldstein SL, Matthews BD, Sing RF, et al. Lateral approach to laparoscopic cholecystectomy in the previously operated abdomen. J Laparoendosc Adv Surg Tech A 2001;11:183-6. [Crossref] [PubMed]
- Bouasker I, El Ouaer MA, Smaali I, et al. Laparascopic cholecystectomy on a previously operated abdomen. Tunis Med 2010;88:88-91. [PubMed]
- Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg 2010;145:19-23. [Crossref] [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [Crossref] [PubMed]
- Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761-8. [Crossref] [PubMed]
- Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. [Crossref] [PubMed]
- Diez J, Delbene R, Ferreres A. The feasibility of laparoscopic cholecystectomy in patients with previous abdominal surgery. HPB Surg 1998;10:353-6. [Crossref] [PubMed]
- Tsunoda S, Okabe H, Obama K, et al. Laparoscopic gastrectomy for patients with a history of upper abdominal surgery: results of a matched-pair analysis. Surg Today 2014;44:271-6. [Crossref] [PubMed]


