Prognostic significance of the platelet-to-lymphocyte ratio in ovarian cancer: a meta-analysis
Introduction
Ovarian cancer accounts for 3.6% of all malignancies in females globally, which is the leading cause of cancer-related mortality among gynecological malignancies (1). In 2016, an estimated 22,280 patients were predicted to be newly diagnosed with ovarian cancer in the United States, of whom, 14,240 were predicted to die of the disease (1). Approximately 70% of ovarian cancer patients are diagnosed at an advanced stage, and only 40% of them are expected to survive more than 5 years (2,3). Primary cytoreductive surgery alone or in combination with adjuvant chemotherapy is the standard treatment regimen for ovarian cancer. Despite an initial good response to chemotherapy, almost 75% of these patients will ultimately recur and die of the disease (4), accounting for the major reason for cancer-related death. Therefore, reliable and available prognostic indicators would alert surgeons about the strengthened necessity of follow-up for these high-risk patients. Additionally, an earlier observation and earlier therapy would benefit these patients.
At present, the prognostic indicators for ovarian cancer are as follows: performance status, age at diagnosis, International Federation of Obstetricians and Gynecologists (IFGO) tumor stage, histological classification, preoperative molecular markers, tumor grade as well as presence of a residual disease after the initial surgery (5). Preoperative molecular markers, including serum human kallikreins, plasma D-dimer, serum CA-125, serum vascular endothelial growth factor (VEGF), serum cytokines as well as soluble cytokeratin fragments, are prognostic variables for ovarian cancer (6,7). However, the application of the above biomarkers has two main drawbacks. First, they require tissue samples, which may not be available for every patient, especially for those patients harboring smaller tumors. Second, other factors may influence the results of the immunohistochemical assay, such as the quality of the antibody (8). Therefore, there is a clinical need for simple, easily available biomarkers.
The systemic inflammatory response (SIR) is a crucial and essential process during carcinogenesis and tumor progression. Inflammation is closely associated with cancer initiation, promotion, malignant conversion, invasion and metastasis (9-12). The inflammatory response to a tumor is mediated by neutrophils, lymphocytes and other phagocytic mediators, thereby suppressing apoptosis, inducing cellular DNA damage and enhancing angiogenesis around the cancerous region. In a similar pattern, platelets can generate and release certain growth factors [e.g., platelet-derived growth factor (PDGF), thrombospondin, transforming growth factor beta, platelet factor 4 as well as VEGF], which are considered to act as strong mitogens or adhesive glycoproteins for diverse types of cells. Several inflammatory biomarkers that are routinely available from pretreatment routine blood tests, such as platelet count, neutrophil to lymphocyte ratio (NLR) as well as platelet-to-lymphocyte ratio (PLR), have been used to assess the prognosis of various types of cancers (13-17).
The peripheral blood PLR, measured during the preoperative or pretreatment phase, is an independent predictor of poor prognosis in multiple malignancies, including lung, breast, pancreatic, colon and gastric cancers (18-22). Nevertheless, it remains largely unknown of preoperative PLR in the prognosis in patients with ovarian cancer. Therefore, this study was designed to assess the association between preoperative PLR values and prognosis in ovarian cancer patients.
Methods
This analysis was conducted in accordance with PRISRMA guidelines.
Data sources and search strategies
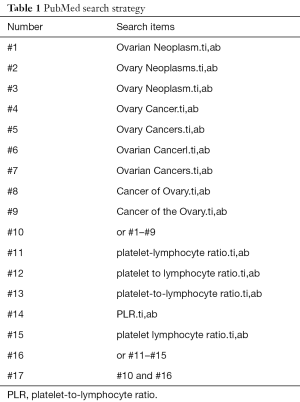
A systematic review of studies concerning the application of PLR for predicting the prognosis of ovarian cancer was performed. We electronically searched the following databases: Medline (host: OVID), including studies from 1946 to April 2017; Embase (host: OVID), including studies from 1974 to April 2017; and Web of Science and Cochrane Database of Systematic Reviews, including studies from 2005 to June 2017. The following search terms were used for the database searches: PLR, platelet-lymphocyte ratio, platelet lymphocyte ratio or PLR with ovary neoplasm, ovary neoplasms, ovarian neoplasm, ovarian cancer, ovarian cancers, ovary cancer, ovary cancers, cancer of the ovary and cancer of ovary. Free text as well as Mesh search for keywords were employed. The search strategy utilized in the PubMed database was shown in Table 1, which was also applied to other electronic databases.
Full table
Study selection
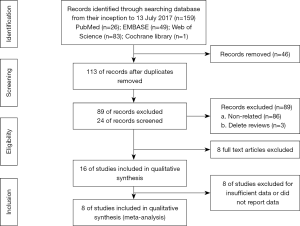
The search was performed by two investigators (Xu and Wang), who read the titles and abstracts of all candidate literature. Full-text was retrieved for review in the case of failure in categorization of the articles simply based on the title and abstract, the. The articles were checked and read independently in accordance with the inclusion criteria in this study. Any divergence during the selection period was discussed and decided on by a third investigator (Yang). The PRISMA flowchart showed the details of the selection process (Figure 1).
Inclusion and exclusion criteria
Eligible studies were enrolled in line with the following criteria: (I) researched patients who underwent operations with any type of ovarian cancer; (II) explored the correlation of the pretreatment PLR with overall survival (OS) and progression-free survival (PFS); and (III) presented in a full paper published in English. The exclusion criteria were as follows: (I) letters, case reports, reviews or laboratory studies; (II) studies with repeated analysis or duplicate data; (III) studies without necessary data for further analysis; or (IV) non-human studies.
Data extraction
Predesigned extraction forms were used to collect the following data from each study: the first author’s name, number of patients included in the study, country of origin of the patients, year of publication, therapeutic methods, cut-off value, HR of the PLR for the OS with its 95% confidence intervals (CIs) and p value, and HR of the PLR for the PFS with its 95% CIs and p value. Assuming that most deaths would be disease-related, in the case of inaccessible data about OS, cancer-specific survival (CSS) information was obtained instead. The accessible HRs were obtained from multivariable analyses, while the HRs from univariable analyses were extracted or estimated from Kaplan-Meier curves, as proposed by Parmar and colleagues. If available, we also collected the HRs for survival associated with C-reactive protein, the NLR and Glasgow prognostic score (GPS) or modified GPS. The HRs for subgroups were compared as defined by different markers in order to assess the relative prognostic effect of PLR with other inflammatory factors.
Data synthesis and statistical analyses
The HRs and 95% CIs were directly acquired from each study publication. In the case of indirect data, mathematical estimations were performed by calculating the necessary data in accordance with specific method. If a meta-analysis could be performed, STATA software version 12.0 (STATA Corporation, College Station, TX, USA) was employed to combine the HR with the 95% CIs for dichotomous outcomes and the weighted mean difference or standardized mean difference with 95% CIs for continuous data. All statistical tests were bilateral, and P<0.05 was considered significant. If the data were not suitable for combining quantitatively, we performed a systematic narrative synthesis with available data in the text to explain and summarize the findings and characteristics of enrolled studies.
Heterogeneity analysis
The Cochran’s Q test as well as Higgins I-squared statistic were used to determine the heterogeneity of pooled outcomes. A P value <0.05 for heterogeneity and/or an I-squared statistic >50% showed significant heterogeneity, where the random-effects model (DerSimonian-Laird method) was used to combine the data, otherwise, the fixed-effect model (Mantel-Haenszel method) was employed. In addition, we performed a subgroup analysis by enrolling variables such as the PLR cut-off value, ethnicity, and therapeutic method, aiming at determination of the potential source of heterogeneity among studies.
Assessment of the qualities of the studies
Newcastle-Ottawa Scale (NOS) was employed to determine the qualities of enrolled studies (23), including selection, comparability as well as outcomes, with a maximal score of 9. Studies were regarded as high quality with scores of or over 7.
Sensitivity analysis
If the P value from the heterogeneity test was under 0.05 after data extraction, the study was checked, followed by subgroup analyses. In addition, a sensitivity analysis was conducted to verify the convincingness of outcomes in this meta-analysis by sequentially omitting each individual study using the ‘‘metaninf’’ STATA command.
Assessment of publication biases
The Begg’s funnel plot as well as the Egger’s linear regression test were performed to evaluate publication biases. A P<0.05 was considered to be statistical significance.
Results
Search results and study characteristics
Initially, 159 studies were collected from electronic databases. After removing duplicates and inspecting titles and/or abstracts, 16 full-text articles were further assessed. Of them, eight studies including 1,636 subjects were ultimately included in this study (14,24-30) after eliminating the other eight studies due to the insufficient or absent data concerning PLR. The search steps in detail were shown in Figure 1. The median sample size consisted of 205 patients, ranging from 30 to 344 patients. Seven studies were conducted in Asian countries, and one study was performed in a non-Asian country. And the cut-off values for the PLR varied from 129.78 to 300. The association between the PLR and OS was investigated in all the eight studies, while that of the PLR with PFS was explored in five studies. In all the eight studies, the NOS scores were over 6. In addition, the baseline characteristics of the eight enrolled studies were summarized in Table 2.

Full table
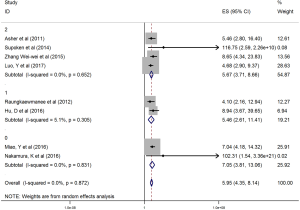
Impact of the PLR on OS and DFS in ovarian cancer patients
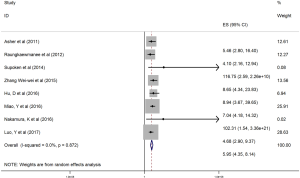
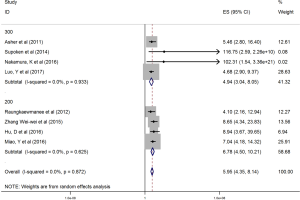
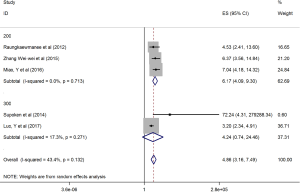
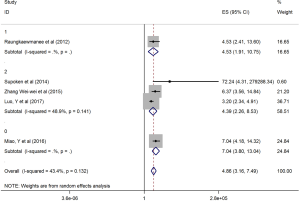
The HRs along with 95% CIs from the 1,636 patients from the eight studies were extracted and pooled. Consequently, there was a significant correlation between the PLR and worse OS (HR =5.95, 95% CI: 4.35–8.14, P=0.000, Figure 2) and that the heterogeneity was not significant (I2 =0.0%, P=0.872, Figure 2). The random effect model was utilized, although both models could be used. Moreover, subgroup analysis was performed for further investigation, and the PLR cut-off value remained an indicator of poor OS near 200 (HR =6.78, 95% CI: 4.50–10.21, P<0.001, Figure 3) and near 300 (HR =4.94, 95% CI: 3.04–8.05, P<0.001, Figure 3). Additionally, the PLR remained a significant prognostic indicator for OS of subjects undergoing mixed therapy (HR =5.67, 95% CI: 3.71–8.66, P=0.000, Figure S1), chemotherapy (HR =7.05, 95% CI: 3.81–13.06, P=0.000, Figure S1) or surgery (HR =5.46, 95% CI: 2.61–11.41, P=0.000, Figure S1). As we had mentioned, the prognostic value of the PLR on PFS was reported in five studies with 1,268 subjects. Consequently, there existed a significant correlation between the PLR and was worse PFS (HR =4.86, 95% CI: 3.16–7.49, P<0.001, Figure S2), with insignificant heterogeneity (I2 =43.4%, P=0.132, Figure S2). Further, subgroup analysis showed that the PLR cut-off value remained an indicator for poor PFS near 200 (HR =6.17, 95% CI: 4.09–9.30, P<0.001, Figure S3) but not near 300 (HR =4.24, 95% CI: 0.74–24.46, P>0.05, Figure S3). Additionally, the PLR remained a significant prognostic factor for the PFS of subjects undergoing mixed therapy (HR =4.39, 95% CI: 2.26–8.53, P=0.000, Figure S4), chemotherapy (HR =7.04, 95% CI: 3.80–13.04, P=0.000, Figure S4) or surgery (HR =4.53,95% CI: 1.91–10.75, P=0.000, Figure S4). Together, the above-described findings indicate that an elevated PLR was significantly related to poor OS and PFS in ovarian cancer patients.
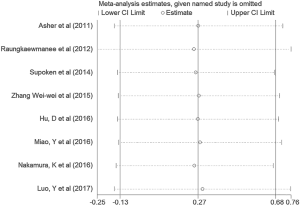
Sensitivity analysis
We performed the sensitivity analyses by the sequential omission of each individual study, aiming to determine whether the outcomes were affected by any individual study. This analysis indicated no obvious effect on the pattern of the results by any single study (Figure 4).
Publication bias
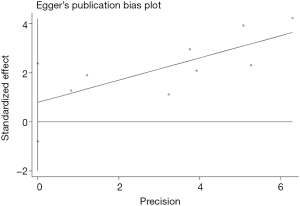
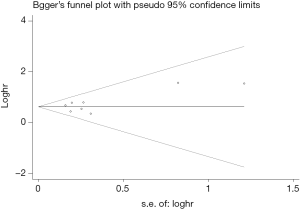
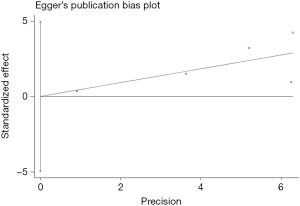
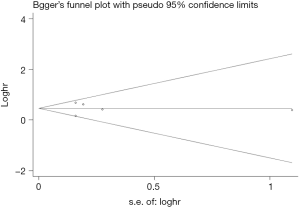
Begg’s funnel plot as well as Egger’s linear regression test were conducted to assess the possible publication bias of our study. As a result, there was no significant publication bias, as indicated by P values for the OS of 0.269 (Egger’s test; Figure S5) and 0.269 (Begg’s test; Figure S6), and P values for the PFS of 0.243 (Egger’s test; Figure S7) and 0.243 (Begg’s test; Figure S8).
Discussion
In this meta-analysis enrolling eight studies, the PLR was a significant biomarker for poor OS and PfS The subgroup analysis demonstrated that the PLR cut-off value was an indicator for poor OS near 200 and near 300. In addition, the PLR was also a significant prognostic indicator for the OS in subjects undergoing anti-cancer therapy, including those receiving a mixed treatment of chemotherapy and surgery.
To date, a variety of predictors, such as the TNM stage, CA-125, as well as inflammatory factors, have been confirmed and subsequently applied to the prognostic prediction of ovarian carcinoma (5). SIRs have been shown to boost tumor progression at almost each single step, such as initiation, progression as well as distant metastasis (31). Chemokines as well as inflammatory cytokines could be secreted by both tumor cells and host cells (including leukocytes and platelets), rendering malignant progression (32). However, it remains largely unknown of the specific mechanism of this progression. An inflammatory process triggered by cancer cells could be used to explain the association of poor prognosis with elevated platelets, lymphocytes or their ratio. On the one hand, thrombocytosis is commonly detected in ovarian cancer patients, which is associated with poor survival (33). Platelets can promote tumor growth, angiogenesis and metastasis by secreting a variety of growth factors, including PDGF, platelet-activating factor, and VEGF (34). Moreover, platelets are capable of facilitating tumor cell transendothelial migration and metastasis by mediating the P2Y2 receptor. The survival of ovarian cancer patients is negatively influenced by elevated platelet levels. On the other hand, lymphocytes are critically involved in cancer immune-surveillance to prevent tumor development (35). Lymphocytes exert an anti-tumor effect via induction of cytotoxic cell death as well as suppression of tumor proliferation (31). Hence, the survival is relatively better in cancer patients harboring enhanced infiltration of lymphocytes into tumor tissue (36).
Multiple previous researches have suggested that elevated PLR is associated with poor survival for patients harboring different malignancies, such as NSCLC (37), pancreatic cancer (38,39), breast cancer (40), based on meta-analyses. However, other studies have found that the PLR was a negative prognostic factor for pancreatic ductal adenocarcinoma (38,39), hepatocellular carcinoma (41) and colorectal cancer (42). Gu (43) found that the PLR failed to be a significant indicator for the OS of gastric cancer patients. However, there has been no meta-analysis concerning about the prognostic significance of the PLR in ovarian cancer patients. To our knowledge, our study is the first meta-analysis to probe into the association between the PLR and the prognosis of ovarian cancer patients. Consistent with previous studies concerning other types of malignancies, our research demonstrated that there was a significant correlation of elevated PLR with poor OS as well as PFS in ovarian cancer patients. In addition, we also revealed that the PLR could be utilized as a poor prognosis factor and a potential significant biomarker for the OS in ovarian cancer patients. Therefore, we suggest that the PLR could be used to predict the prognosis and detect the relapse of ovarian cancer patients.
This study has several limitations. It exclusively included researches in English language, which might lead to publication bias. Moreover, there was relatively large heterogeneity among these studies, which could result from many demographic characteristics, including countries and race, and histological traits, such as histological classification. In order to minimize the heterogeneity in the present study, subgroups were analyzed according to different cut-off values of the PLR, alongside with the heterogeneity analysis, which revealed that the PLR remained a negative factor at different cut-off values. Additionally, the sensitivity analysis showed the same result. Thus, the heterogeneity did not affect the results of our meta-analysis. Moreover, the correlation between the PLR and other clinical and pathological characteristics was not analyzed due to the limited extraction data. Furthermore, most of the patients were classified as Asians in the inclusive studies of our meta-analysis. Therefore, large-scale prospective studies are warranted to provide more convincing outcomes in the future.
Collectively, this meta-analysis indicates that an elevated preoperative PLR is negatively associated with survival of ovarian cancer patients. Additionally, this meta-analysis may provide effective cut-off values for other study groups.

Acknowledgments
Funding: This work was supported by International Science and Technology Cooperation Projects (2015DFA30650 and 2010DFB33720), the Capital Special Research Project for Health Development (2014-2-4012), the Capital Research Project for the Characteristics Clinical Application (Z151100004015170), and the Program for New Century Excellent Talents in University (NCET-11-0288).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Siegel R, Xu J, et al. Cancer Statistics, 2010. CA Cancer J Clin 2010;60:277-300. [Crossref] [PubMed]
- Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin 2011;61:183. [Crossref] [PubMed]
- Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2009. CA Cancer J Clin 2009;59:225-49. [Crossref] [PubMed]
- Ozols RF. Challenges for chemotherapy in ovarian cancer. Ann Oncol 2006;17:v181-7. [Crossref] [PubMed]
- Gadducci A, Cosio S, Tana R, et al. Serum and tissue biomarkers as predictive and prognostic variables in epithelial ovarian cancer. Crit Rev Oncol Hematol 2009;69:12-27. [Crossref] [PubMed]
- Jatoi A, Vierkant RA, Hawthorne KM, et al. Clinical and emergent biomarkers and their relationship to prognosis of ovarian cancer. Oncology 2016;90:59-68. [Crossref] [PubMed]
- Yang ZJ, Zhao B, Li L. The significance of the change pattern of serum CA125 level for judging prognosis and diagnosing recurrences of epithelial ovarian cancer. J Ovarian Res 2016;9:57. [Crossref] [PubMed]
- Au KK, Josahkian JA, Francis JA, et al. Current state of biomarkers in ovarian cancer prognosis. Future Oncol 2015;11:3187. [Crossref] [PubMed]
- Dreyer SB, Powell AG, Mcsorley ST, et al. The Pretreatment Systemic Inflammatory Response is an Important Determinant of Poor Pathologic Response for Patients Undergoing Neoadjuvant Therapy for Rectal Cancer. Ann Surg Oncol 2017;24:1295-303. [Crossref] [PubMed]
- Geng Y, Qi Q, Sun M, et al. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur J Surg Oncol 2015;41:1508-14. [Crossref] [PubMed]
- Namikawa T, Munekage E, Munekage M, et al. Evaluation of Systemic Inflammatory Response Biomarkers in Patients Receiving Chemotherapy for Unresectable and Recurrent Advanced Gastric Cancer. Oncology 2016;90:321-6. [Crossref] [PubMed]
- Zhu L, Li X, Shen Y, et al. A new prognostic score based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Onco Targets Ther 2016;9:4879-86. [Crossref] [PubMed]
- Asher V, Lee J, Bali A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med Oncol 2012;29:2005. [Crossref] [PubMed]
- Asher V, Lee J, Innamaa A, et al. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol 2011;13:499-503. [Crossref] [PubMed]
- Hefler LA, Concin N, Hofstetter G, et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res 2008;14:710-4. [Crossref] [PubMed]
- Petri AL, Høgdall E, Christensen IJ, et al. Preoperative CA125 as a prognostic factor in stage I epithelial ovarian cancer. Apmis 2006;114:359-63. [Crossref] [PubMed]
- Williams KA, Labidi-Galy SI, Terry KL, et al. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol 2014;132:542-50. [Crossref] [PubMed]
- Chen XD, Mao C, Wu R, et al. Use of the combination of the preoperative platelet-to-lymphocyte ratio and tumor characteristics to predict peritoneal metastasis in patients with gastric cancer. PLoS One 2017;12:e0175074 [Crossref] [PubMed]
- Krenn-Pilko S, Langsenlehner U, Thurner EM, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer 2014;110:2524-30. [Crossref] [PubMed]
- Qiang G, Liang C, Fei X, et al. Prognostic significance of platelet-to-lymphocyte ratio in non-small-cell lung cancer: a meta-analysis. Onco Targets Ther 2016;9:869-76. [Crossref] [PubMed]
- Shirai Y, Shiba H, Haruki K, et al. Preoperative Platelet-to-Albumin Ratio Predicts Prognosis of Patients with Pancreatic Ductal Adenocarcinoma After Pancreatic Resection. Surgery 2015;158:360-5. [Crossref] [PubMed]
- Szkandera J, Pichler M, Absenger G, et al. The elevated preoperative platelet to lymphocyte ratio predicts decreased time to recurrence in colon cancer patients. Am J Surg 2014;208:210. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, et al. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol 2012;23:265-73. [Crossref] [PubMed]
- Supoken A, Kleebkaow P, Chumworathayi B, et al. Elevated Preoperative Platelet to Lymphocyte Ratio Associated with Decreased Survival of Women with Ovarian Clear Cell Carcinoma. Asian Pac J Cancer Prev 2014;15:10831-6. [Crossref] [PubMed]
- Zhang WW, Liu KJ, Hu GL, et al. Preoperative platelet/lymphocyte ratio is a superior prognostic factor compared to other systemic inflammatory response markers in ovarian cancer patients. Tumour Biol 2015;36:8831-7. [Crossref] [PubMed]
- Nakamura K, Nagasaka T, Nishida T, et al. Neutrophil to lymphocyte ratio in the pre-treatment phase of final-line chemotherapy predicts the outcome of patients with recurrent ovarian cancer. Oncol Lett 2016;11:3975-81. [Crossref] [PubMed]
- Luo Y, Kim HS, Kim M, et al. Elevated plasma fibrinogen levels and prognosis of epithelial ovarian cancer: a cohort study and meta-analysis. J Gynecol Oncol 2017;28:e36 [Crossref] [PubMed]
- Hu D, Lin Y, Liu F, et al. Elevated Preoperative Platelet to Lymphocyte Ratio Indicates Poor Survival in Patients with Resected High-grade Serous Ovarian Carcinoma. Clin Lab 2016;62:1443-9. [Crossref] [PubMed]
- Miao Y, Yan Q, Li S, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are predictive of chemotherapeutic response and prognosis in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Cancer Biomark 2016;17:33-40. [Crossref] [PubMed]
- Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol 2013;33:S79-84. [Crossref] [PubMed]
- Balkwill F, Mantovani A, Balkwill F, et al. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [Crossref] [PubMed]
- Ikeda M, Furukawa H, Imamura H, et al. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol 2002;9:287-91. [Crossref] [PubMed]
- Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost 2011;9:237-49. [Crossref] [PubMed]
- Dunn GP, Old LJ, Schreiber RD, et al. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity 2004;21:137-48. [Crossref] [PubMed]
- Nguyen N, Emily B, Daffyd T, et al. Walline H and Jeffery Moyer MD. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck 2016;38:1074-84. [Crossref] [PubMed]
- Zhao QT, Yuan Z, Zhang H, et al. Prognostic role of platelet to lymphocyte ratio in non-small cell lung cancers: A meta-analysis including 3,720 patients. Int J Cancer 2016;139:164-70. [Crossref] [PubMed]
- Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2014;23:1204-12. [Crossref] [PubMed]
- Zhou X, Du Y, Huang Z, et al. Prognostic Value of PLR in Various Cancers: A Meta-Analysis. PLoS One 2014;9:e101119 [Crossref] [PubMed]
- Zhu Y, Wen S, Sun Q, et al. Platelet-lymphocyte ratio acts as an indicator of poor prognosis in patients with breast cancer. Oncotarget 2017;8:1023-30. [PubMed]
- Zhao Y, Si G, Zhu F, et al. Prognostic role of platelet to lymphocyte ratio in hepatocellular carcinoma: a systematic review and meta-analysis. Oncotarget 2017;8:22854-62. [Crossref] [PubMed]
- Tan D, Fu Y, Su Q, et al. Prognostic role of platelet-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e3837 [Crossref] [PubMed]
- Gu X, Gao XS, Cui M, et al. Clinicopathological and prognostic significance of platelet to lymphocyte ratio in patients with gastric cancer. Oncotarget 2016;7:49878-87. [Crossref] [PubMed]