
Galectin-9 as a prognostic biomarker in small cell lung cancer
Introduction
Small cell lung cancer (SCLC) is a deadly tumor that is a highly aggressive and widely metastatic cancer, with a 7% 5-year survival rate (1). SCLC is one pathology type of lung cancer which also includes lung adenocarcinoma, lung squamous carcinoma and large cell lung cancer. Although SCLC accounts for approximately 15% of lung cancer patients, it has the poorest prognosis of all lung cancers. Although there has been much effort in conducting comprehensive molecular research (2), there have been no important therapeutic clinical advances for 30 years, leading SCLC to be designated as an intractable cancer (1). Furthermore, SCLC is a neuroendocrine tumor which has a relatively complex tumor microenvironment. Recently, immune checkpoint inhibitors play an important role in anti-tumor therapies for melanoma and renal cell carcinoma, bladder cancer, non-small cell lung cancer and others (3,4). However, there have been few studies to analyze the functions of immune checkpoints in the progression of SCLC.
The Galectin family has many biological functions, such as immunological modification, tumor cell metastasis, the induction and maintenance of tumor angiogenesis, and so on (5). Galectin-9 is one member of the galectin family and is mainly expressed on macrophagocytes, antigen-pressed cells and tumor cells, and it can ligand with T-cell immunoglobulins and mucin-domain containing-3 (TIM-3) which is expressed on lymphocytes so as to inhibit its function (6,7). Blocking the ligation between galectin-9 and TIM-3 may partially recover the function of the exhausted lymphocytes (8,9). However, there are many different, even contradictory results about the survival analysis of galectin-9 in different cancers.
In this study, we evaluated the expression of galectin-9 in clinical specimens of 48 SCLC patients by immunohistochemical analysis (IHC). The association between galectin-9 expression and clinicopathological characteristics, and overall survival (OS) by the Kaplan-Meier curve and cox survival analysis were also elucidated. Moreover, we evaluated the predictive potential of galectin-9 expression in SCLC patients with chemotherapy or radiotherapy.
Methods
Patients and specimens
This study was approved by the Shengjing Hospital of Chinese Medical University ethics committee (No. 2016PS256K), and we acquired exemptions via informed consent from patients for the use of abandoned paraffinic specimens. We collected pathological specimens from 48 patients with SCLC who underwent surgery at the China Medical University affiliated Shengjing Hospital, China, from 2008 to 2014, though three patients failed to follow-up. Most of the SCLC patients that received surgery by mistake did not carry out an effective pathological examination or puncture before surgery. And the nodules of minor established SCLC patients could have been resected, such patients were in the 1A stage (T1N0M0 in TNM stage) and not infiltrated in visceral pleura, with main bronchus, surrounding lymph nodes or distant organs and tumor sizes of less than 3 centimeters. Patients were diagnosed in stages by serum tumor markers: CEA, CY21-1, NSE, SCC; and imaginology examinations, positron emission tomography/computed tomography (PET/CT) or chest CT scans, bone ECT, and brain contrast-enhanced magnetic resonance imaging were conducted before surgery. The mean age of these patients was 56.6 years, with a range of 33 to 82 years. Most of the included SCLC patients had received etoposide and cis-platinum (EP) chemotherapy, and some received etoposide and lobaplatin, epirubicin and cyclophosphamide (EC), cyclophosphamide, adriamycin and vincristine (CAV) and irinotecan and paraplatin (IP). Patients who had received neoadjuvant therapy or immune system related diseases were excluded. Histologic diagnoses were based on hematoxylin and eosin staining according to the World Health Organization 2004 criteria. We used neoplastic and paraneoplastic specimens (more than 5 centimeters apart from margin of the tumor) to carry out the following research.
Neuron specific enolase (NSE) is one of the most important markers used to evaluate the progress of SCLC patients (10). SCLC are patients always accompanied with hyponatremia, which is caused by SIADH (inappropriate secretion of antidiuretic hormone) or paraneoplastic syndrome and so on, and hyponatremia always predicts a poor outcome. Meanwhile, some SCLC patients with a state of hypercoagulability (high level D-dimer) also had worse prognoses. Among the above-mentioned data, NSE, Na+, and D-dimer were acquired in the serum of patients the day after their admission to the hospital, always about one week before surgery. Clinicopathological data collected for analysis included sex, tumor location, age of diagnosis, tumor size, node involvement (N), NSE expression, Na+ (sodium ion) level and D-dimer expression. Disease recurrence and survival were observed during the follow-up clinic or obtained through telephone correspondence. Follow-up was until death or December 2015.
Immunohistochemistry
IHC was performed on the resected SCLC tumor tissues using the primary antibodies, anti-galectin-9 antibody (1:800, 54330, CST) and CD8 (1:100, 17335-1-AP, Proteintech Group, China), according to a previously described protocol (11). All results were recorded by NIS-Elements F 3.0 (Japan) and computerized image systems (Nikon E800 microscope, Japan). All IHC results were analyzed by two pathologists independently in a blind manner. The intensity for staining was defined as follows: no staining was considered a negative result (“0”). Other positively stained sections were analyzed by color: weak staining (“1”), moderate staining (“2”), strong staining (“3”). This was followed by a calculation of the histoscore (H-score) according to the following formula: H-score=1*(%cell “1”) +2*(%cell “2”) +3*(%cell “3”). A mean H-score was obtained by averaging these five representative fields (400× objective).
Statistical analysis
We carried out correlations between IHC expression and the clinic-pathological features by the Pearson’s chi-squared test, and calculated survival situations of different groups by the Kaplan-Meier survival and log-rank tests. And Cox regression model was used to carry out multivariate analysis. The statistical analyses were performed using the SPSS software program, version 20.0 (SPSS, Chicago, IL, USA), which was considered statistically significant when P values are less than 0.05.
Results
The expression level and correlation of galectin-9 and clinic-pathological features
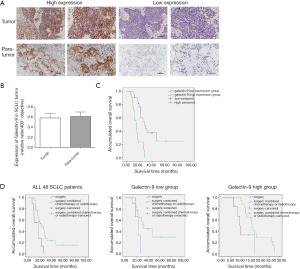
We evaluated galectin-9 expression in 48 human SCLC tissues by IHC. First, galectin-9 was abundant and expressed mainly in the cytoplasm and membrane of cancer cells (Figure 1A). There was no obvious difference between the galectin-9 expression of tumor tissue and para-tumor tissues, including bronchial alveolar cells and lymphoid tissues (Figure 1B). There were 24 SCLC patients over cut-off values, forming the galectin-9-high expression group, and 24 SCLC patients below cut-off values, forming the galectin-9-low expression group. Detailed clinic-pathological characteristics are shown in Table 1. We found that over-expressed galectin-9 on tumor specimens was associated with high levels of NSE expression (P=0.04). In this way, we thought NSE could be an advantageous factor in the selection of SCLC patients who may benefit more from anti-galectin-9 targeting therapy. However, there was no significant association between over-expressed galectin-9 and tumor size, N, Na+ expression, D-dimer expression or number of CD8+T lymphocytes present in SCLC tissue.

Full table
Prognostic values of galectin-9 in human SCLC
To determine the prognostic value of the expression levels of galectin-9, we used Kaplan-Meier survival calculations and log-rank tests for sex, tumor location, age of diagnosis, tumor size, node status (N), NSE, Na+, D-dimer, postoperative therapeutic method and galectin-9. We found that galectin-9 (P<0.001), NSE (P=0.006) and postoperative therapeutic methods (P=0.006) were associated with patient survival time. As shown in Figure 1C, patients with higher galectin-9 expression (galectin-9-high group) tended to have a shorter overall survival (OS) (15.64±2.32 months) compared to that of the galectin-9-low group (28.13±5.43 months) (P<0.001). Furthermore, we used a multivariate Cox regression model to analyze galectin-9, NSE and postoperative therapeutic methods to determine their prognostic values. In addition to elevated NSE [hazard ratio (HR) =2.27, 95% confidence interval (CI), 1.09–4.70, P=0.03], high galectin-9 expression (HR =6.21, 95% CI, 2.98–12.95, P<0.01) and different therapeutic methods (HR =0.57, 95% CI, 0.33–0.97, P=0.04) were independent predictors of poor overall survival in SCLC (Table 2).

Full table
In the study, 39 of 48 (81.25%) patients received chemotherapy or radiotherapy. To evaluate whether patients with high or low expressed galectin-9 would benefit from chemotherapy or radiotherapy, we studied the relationship between galectin-9 expression and the OS of SCLC patients with different therapeutic methods. We divided the 48 SCLC patients into two groups by galectin-9 cut-off expression into a galectin-9 high group and a galectin-9 low group. In the galectin-9 high expression group, the survival rates from surgery and additional chemotherapy/radiotherapy were a mere 1/6 and 1/17, respectively. In the galectin-9 low expression group, the survival rates from surgery and additional chemotherapy/radiotherapy were 0/3 and 11/19, respectively. There were 3/48 lost during follow-up. We then analyzed the survival of SCLC patients by therapeutic methods (surgery versus a combination of surgery and chemotherapy or radiotherapy). We found patients with chemotherapy or radiotherapy had a longer OS than patients with merely surgery (P=0.006, Figure 1D). There appeared to be an association between the OS of SCLC patients and different therapeutic methods in the galectin-9 high group rather than the galectin-9 low group (Figure 1D). A study about the correlation between galectin-9 expression, therapeutic methods and OS revealed that in the galectin-9 high group, the benefit observed in patients with chemotherapy or radiotherapy was superior to that of those patients without chemotherapy or radiotherapy (P=0.042, Figure 1D). Furthermore, there was no obvious relationship between survival and therapeutic methods in the galectin-9 low group. Therefore, we believe that patients with high galectin-9 expression might benefit more from chemotherapy or radiotherapy after surgery.
Discussion
Lung cancer is one of the deadliest cancers worldwide. In particular, SCLC is the poorest type of lung cancer with an unknown genetic mechanism and high invasion or metastasis. In our study, we revealed the prognostic value of galectin-9 in SCLC. Pathological findings showed that patients with high galectin-9 levels were found to have a higher prevalence of NSE, which is a classic SCLC tumor biomarker. High galectin-9 expression was an independent prognostic factor of the OS of SCLC patients, disregarding NSE and different therapeutic methods. Furthermore, the benefit to patients undergoing chemotherapy or radiotherapy was superior in galectin-9 high expressed SCLC patients.
High-expression of galectin-9 in SCLC patients was found to be related to high NSE levels. On the one hand, NSE could be an advantageous factor in the selection of SCLC patients who may benefit more from anti-galectin-9 targeting therapy. On the other hand, mean NSE values were significantly higher in patients with extensive disease compared to those with limited disease. In this way, we speculate on whether galectin-9 could be related to tumor load, which may be one of the pieces of evidence suggesting that galectin-9 is a bad prognostic biomarker in SCLC.
The results of galectin-9 in survival analysis with various studies were different. Some researchers thought it was a protective factor in breast cancer (12), bladder urothelial carcinoma (13) and melanoma (14) by means of inducing cell aggregation and apoptosis, or decreasing invasion and metastasis. However, others thought it was a disadvantageous risk factor in hepatocellular carcinoma (15), gastric cancer (16), pancreatic carcinoma (17) and lung adenocarcinoma (18) by means of up-regulating the interaction with TIM-3 to decrease the cytotoxic or proliferation of exhausted lymphocytes and so on. As for our results, an elevated galectin-9 expression was an independent disadvantage of a decreased OS in SCLC patients, and we thought this result may partially be related to the complex tumor microenvironment of SCLC. Furthermore, the other members of the galectin family, galectin-1 and galectin-3 probably play a role in the invasion, metastasis, anti-apoptosis and angiogenesis of cancer cells (19). Both galectin-1 and galectin-3 were associated with poor disease outcomes in lung cancer patients as previously reported (5,20).
Recently, there were some studies that found that galectin-9/TIM-3 functions as an immune checkpoint to inhibit the anti-tumor immune functions of lymphocytes, NK cells and so on (13). Zhou et al. found that antibodies against TIM3 restore responses of HCC-derived T cells to tumor antigens (21). Kikushige et al. thought that TIM-3+ leukemic stem cells (LSCs) could also cause autocrine galectin-9 to react with TIM-3 to sustain self-renewal and promote Treg cell differentiation and maintenance (22). Furthermore, some studies have found that the survival time of animals with lung cancer, melanoma and glioma could be prolonged via blocking the reaction of galectin-9/TIM-3 (9,23,24). Aside from this, there have been many studies involved in the study of the immunoregulatory function of galectin-9. Wu et al. found that galectin-9 could react with CD44 to increase iTreg cell stability and function by the transcription factor Smad3, which participated in forming a feed-forward loop (25). In addition, galectin-9 can react with Dectin-1 which is expressed on macrophages and myeloid-monocytic cells to suppress their functions (17). However, Dectin-1 can protect against chronic hepatitis from hepatocarcinoma to induce hepatic fibrosis (26). Galectin-9 can also ligand with CD137 expressed on lymphocytes to activate their functions (27). So, the immuno-regulatory function of galectin-9 is complex and controversial.
In our study, we found that the survival time of patients with chemotherapy or radiotherapy was longer in galectin-9 high SCLC patients, though the survival rate was not obviously increased. Moreover, Liu and colleges also found the benefit associated with adjuvant chemotherapy was superior among galectin-9 low patients than among galectin-9 high patients in bladder urothelial carcinoma (13). We believe this result should be further proved in a larger group. Furthermore, many combination therapies with immune checkpoint inhibitors have been studied recently with great success. Kim et al. found that a combination of anti-TIM-3 on the basis of anti-PD-1 and stereotactic radiosurgery (SRS) could inhibit glioma growth and prolong the survival of mice (23). In this way, whether or not galectin-9 high expressed SCLC patients benefit more from a combination therapy of anti-galectin-9 immune therapy and chemotherapy or radiotherapy should feature in our subsequent studies. We think new immune checkpoint inhibitors with galectin-9 need to be further studied for the prolonging of the survival of SCLC patients.
Conclusions
Our results indicate that elevated galectin-9 expression is an independent indicator of a decreased OS in patients with SCLC. Evaluation of galectin-9 expression may predict the benefits from chemotherapy or radiotherapy in SCLC patients.
Acknowledgments
We are grateful to the pathology department of Shengjing Hospital affiliated to China Medical University in the acquisition of human tumor specimens. We thank members of the Medical Research Center of Shengjing Hospital, China Medical University.
Funding: This work was supported by grants from department of science and technology of Liaoning Province, Liaoning Province Finance Department (2014021032) and Chinese National Natural Science Foundation (61672146).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.18). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Shengjing Hospital of Chinese Medical University ethics committee (No. 2016PS256K), and we acquired exemptions via informed consent from patients for the use of abandoned paraffinic specimens.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer 2017;17:725-37. [Crossref] [PubMed]
- Rudin CM, Poirier JT. Small-cell lung cancer in 2016: Shining light on novel targets and therapies. Nat Rev Clin Oncol 2017;14:75-6. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines®Insights: non–small cell lung cancer, Version 4.2016 Featured Updates to the NCCN Guidelines. J Natl Compr Canc Netw 2016;14:255-64. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Schulkens IA, Heusschen R, van den Boogaart V, et al. Galectin expression profiling identifies galectin-1 and Galectin-9Delta5 as prognostic factors in stage I/II non-small cell lung cancer. PloS One 2014;9:e107988 [Crossref] [PubMed]
- Rangachari M, Zhu C, Sakuishi K, et al. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion. Nat Med 2012;18:1394-400. [Crossref] [PubMed]
- Kikushige Y, Shima T, Takayanagi S, et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell stem cell 2010;7:708-17. [Crossref] [PubMed]
- Zhu S, Lin J, Qiao G, et al. Tim-3 identifies exhausted follicular helper T cells in breast cancer patients. Immunobiology 2016;221:986-93. [Crossref] [PubMed]
- Liu Y, Cai P, Wang N, et al. Combined blockade of Tim-3 and MEK inhibitor enhances the efficacy against melanoma. Biochem Biophys Res Commun 2017;484:378-84. [Crossref] [PubMed]
- Jørgensen LG, Osterlind K, Genollá J, et al. Serum neuron-specific enolase (S-NSE) and the prognosis in small-cell lung cancer (SCLC): a combined multivariable analysis on data from nine centres. Br J Cancer 1996;74:463-7. [Crossref] [PubMed]
- Okita R, Maeda A, Shimizu K, et al. PD-L1 overexpression is partially regulated by EGFR/HER2 signaling and associated with poor prognosis in patients with non-small-cell lung cancer. Cancer Immunol Immunother 2017;66:865-76. [Crossref] [PubMed]
- Irie A, Yamauchi A, Kontani K, et al. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin Cancer Res 2005;11:2962-8. [Crossref] [PubMed]
- Liu Y, Liu Z, Fu Q, et al. Galectin-9 as a prognostic and predictive biomarker in bladder urothelial carcinoma. Urol Oncol 2017;35:349-55. [Crossref] [PubMed]
- Kageshita T, Kashio Y, Yamauchi A, et al. Possible role of galectin-9 in cell aggregation and apoptosis of human melanoma cell lines and its clinical significance. Int J Cancer 2002;99:809-16. [Crossref] [PubMed]
- Li H, Wu K, Tao K, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 2012;56:1342-51. [Crossref] [PubMed]
- Choi SI, Seo KW, Kook MC, et al. Prognostic value of tumoral expression of galectin-9 in gastric cancer. Turk J Gastroenterol 2017;28:166-70. [Crossref] [PubMed]
- Daley D, Mani VR, Mohan N, et al. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat Med 2017;23:556-67. [Crossref] [PubMed]
- Ohue Y, Kurose K, Nozawa R, et al. Survival of Lung Adenocarcinoma Patients Predicted from Expression of PD-L1, Galectin-9, and XAGE1 (GAGED2a) on Tumor Cells and Tumor-Infiltrating T Cells. Cancer Immunol Res 2016;4:1049-60. [Crossref] [PubMed]
- Funasaka T, Raz A, Nangia-Makker P. Galectin-3 in angiogenesis and metastasis. Glycobiology 2014;24:886-91. [Crossref] [PubMed]
- Szoke T, Kayser K, Baumhakel JD, et al. Prognostic significance of endogenous adhesion/growth-regulatory lectins in lung cancer. Oncology 2005;69:167-74. [Crossref] [PubMed]
- Zhou G, Sprengers D, Boor PC, et al. Antibodies against immune checkpoint molecules restore functions of tumorinfiltrating T cells in Hepatocellular Carcinomas. Gastroenterology 2017;153:1107-19.e10. [Crossref] [PubMed]
- Kikushige Y, Miyamoto T, Yuda J, et al. A TIM-3/Gal-9 autocrine stimulatory loop drives self-renewal of human myeloid leukemia stem cells and leukemic progression. Cell Stem Cell 2015;17:341-52. [Crossref] [PubMed]
- Kim JE, Patel MA, Mangraviti A, et al. Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin Cancer Res 2017;23:124-36. [Crossref] [PubMed]
- Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016;7:10501. [Crossref] [PubMed]
- Wu C, Thalhamer T, Franca RF, et al. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity 2014;41:270-82. [Crossref] [PubMed]
- Seifert L, Deutsch M, Alothman S, et al. Dectin-1 regulates hepatic fibrosis and hepatocarcinogenesis by suppressing TLR4 signaling pathways. Cell Rep 2015;13:1909-21. [Crossref] [PubMed]
- Madireddi S, Eun SY, Lee SW, et al. Galectin-9 controls the therapeutic activity of 4-1BB-targeting antibodies. J Exp Med 2014;211:1433-48. [Crossref] [PubMed]


