
Antineoplastic effects of Endostar combined with combretastatin A4 phosphate in vitro
Introduction
The process of tumor angiogenesis in tumor growth and metastasis, which ultimately determine the overall survival of cancer patients, is complex and essential. Vascular targeting agents are now divided into two types, vascular disrupting agents (VDAs) and angiogenesis inhibitors (AIs). VDAs target the pre-existing tumor vasculature causing rapid vascular shutdown leading to cell death and central necrosis (1). AIs prevent the blood vessel formation.
Combretastatin A4 phosphate (CA4P), a tubulin-binding VDA, is a water-soluble prodrug of combretastatin A4 (2). CA4P exhibits selective and potent toxicity targeting tumor vasculature (2,3). The cutoff of blood and oxygen supply collapses the tumor vasculature and thus causes hemorrhagic necrosis in tumors (4,5). Active combretastatin A4 can be obtained through the hydrolysis of CA4P by nonspecific endogenous phosphatases in the plasma (6). The tumoricidal effect of CA4P has been evaluated on a wide variety of tumor models (7,8). In addition, the therapeutic effects of a range of tumor treated by CA4P combined with conventional chemotherapy and radiotherapy were analyzed (9). It has an acceptable toxicity profile in advanced non-small cell lung cancer associated with combination therapy of CA4P with carboplatin, paclitaxel, and bevacizumab (10). CA4P is currently under investigation in phase II trials for the treatment of ovarian, lung, and anaplastic thyroid cancer (11,12). Moreover, antitumor effects could be enhanced in animals in the combined CA4P and bevacizumab treatment and are on the stage of clinical trial evaluation (13,14). Our previous study reported that the combination of CA4P and Endostar had antitumor synergy in an osteosarcoma xenograft and were comparable to adriamycin, the current gold standard of osteosarcoma chemotherapy (15).
Angiogenesis plays a key role in tumor growth and metastasis (16,17). Therefore, anti-angiogenesis therapy has become a vital part of therapeutic strategy. Endostar is a kind of anti-angiogenesis agent that have a broad spectrum of activity against solid tumors and was approved by the Chinese State Food and Drug Administration in 2005 as a specific drug for the treatment of non-small cell lung cancer (18). Endostatin is a 20 kDa endogenous protein proven to inhibit tumor growth and angiogenesis in animal models (19). Endostar, as a recombinant human endostatin, was shown to be a novel anti-angiogenesis agent (20-23) that effectively inhibits tumor angiogenesis and endothelial cell proliferation. However, similar to most protein-based drugs, Endostar has short biological half-life because of rapid metabolism (23).
The main objective of this study aimed to evaluate the effect of CA4P in combination with Endostar as a potential therapeutic option. Our hypothesis lies in our previous published study that the combination of CA4P and Endostar had antitumor synergy in osteosarcoma cells. Subsequently, our study assessed the effect of CA4P and/or Endostar on K562, K562/ADR, MNNG/HOS, MCF-7, T-47D, HT29 cells. The effect of Endostar showed two basic categories: synergistic effect on MNNG/HOS, K562/ADR, and T-47D cells and additional effect on HT29, K562, and MCF-7 cells. For this purpose, MNNG/HOS cells and HT29 cells are chosen to further detect apoptosis, necrosis, synergistic cytotoxicity, cell cycle block and expression of apoptosis-related gene mRNA using different molecular biology techniques under the treatment of CA4P and/or Endostar.
Methods
Cell lines and reagents
Human osteosarcoma cell line MNNG/HOS, Human colon cancer cell line HT29, Human chronic myeloid leukemia cell line K562 and K562/ADR, Human breast cancer cell line MCF-7, Human breast cancer cell line T-47D, were provided by our cancer institute. CA4P was kindly provided by Professor Zou, School of Pharmaceutical Sciences, Sun Yat-sen University. Endostar was provided by the Shandong Simcere Medgenn Bio-Pharmaceutical Co., Ltd., Nanjing, China.
Cell culture
Human osteosarcoma cell line MNNG/HOS were cultured in Eagle’s Minimal Essential Medium (EMEM) medium (GIBCO-BRL, NY, USA). Human breast cancer cell line T-47D were cultured in DMEM medium (GIBCO-BRL, NY, USA). Other cell lines were cultured in RPMI-1640 medium (GIBCO-BRL, NY, USA). Drug-resistant cell K562/ADR with high expression of P-gp were cultured in medium containing 200 ng/mL adriamycin to maintain drug resistance. And drug-resistant cell lines entered to the withdrawal a week before detection of drug-resistant and then were cultured in RPMI-1640 medium. All kinds of cell lines were cultured in medium containing 10% fetal calf serum and 1:100 penicillin and streptomycin in an incubator (37 °C, 5% CO2). Cells were passaged in a 1–3 ratio to a 1–5 ratio every 2 to 3 days.
MTT assay
MTT assay was used to assess the effect of CA4P and/or Endostar on cell proliferation. Briefly, cells were seeded in 96-well plates adding corresponding drugs for different periods of time (12, 24, 48, and 72 h). Then cells were incubated at 37 °C with 20 uL of MTT (5 mg/mL) each well for 4 h and then centrifuged and the supernate was discarded. The cells were replenished with 120 µL dimethyl sulfoxide (DMSO) per hole, after which the absorbance at 570 nm was measured using a microplate reader (Bio-Tek Instruments, Inc., USA). The above experimental procedures were repeated three times. The cell viability rate was calculated by using the following formula: cell viability rate (%) = (OD value after adding with drugs − OD value of the blank)/(OD value of the contrast − OD value of the blank) ×100%. Cell inhibition rate (%) = 1 − cell viability rate(%). The drug concentrations and the cell inhibition rate were drawn using GraphPad Prism 5 software.
Cell cycle and apoptosis analysis
Cells (1×106) were washed by phosphate-buffered saline (PBS) twice and Immobilized with 70% cool ethanol at 4 °C 24 h after the addition of CA4P and/or Endostar, after which they were dyed with Annexin V and propidium iodide (PI). Cell cycle was measured using flow cytometry and ModFit 3.0 software. The percentage of apoptotic cells was measured by applying the criteria of Annexin V-positivity and PI-negativity, while the percentage of late apoptotic cells was determined by applying the criteria of Annexin V-positivity and PI-positivity.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis
Total cellular RNA was obtained using Trizol reagent (Invitrogen, USA). Complementary DNA (cDNA) was synthesized from 2 µg of total RNA using M-MLV reverse transcriptase (Promega, USA). qRT-PCR was performed using StepOnePlus™ Real-Time PCR Systems (Biosystems, UK) with SYBR Green PCR Master Mix (Biosystems, USA). The PCR reaction system consisted of 0.25 µL of Taq enzyme (Dalian Takara Biomedical Technology Co., Ltd., China), 1 µL of each primer, 1 µL of cDNA template, 5 µL of 10× buffer, 4 µL of dNTP and 37.75 µL of ddH2O. The primer sequences are listed in Table 1.
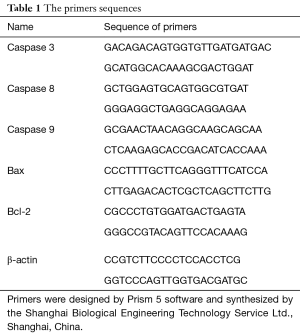
Full table
Statistical analysis
The data of non-normal distribution were obtained using ANOVA with Tukey’s test as post-test and t-test. The data of normal distribution were obtained with nonparametric Kruskal Wallis test. The results were expressed as mean ± standard deviation (SD). P value of less than 0.05 was considered to be a significant. Statistical analyses were carried out in SPSS (version 16.0). Graphical analysis was performed using GraphPad Prism 5.
Results
Effect of CA4P or Endostar on six cell lines
The growth inhibition under the effects of CA4P and/or Endostar was assessed using six cell lines, namely, K562, K562/ADR, MNNG/HOS, MCF-7, T-47D, HT29 cells. The IC50 of CA4P alone on MNNG/HOS cells is 8.72×10−3 µM. Whereas the IC50 of CA4P combined with Endostar is non-detected. It indicates that the combination of Endostar and CA4P has the effect of synergistic cytotoxicity on MNNG/HOS cells and is not limited to anti-angiogenic effects. It demonstrates that Endostar and CA4P synergistically interact against both K562/ADR and T-47D cells alike. In contrast, the IC50 of CA4P alone on HT29 cells is 5.86 µM, whereas the IC50 of combined Endostar and CA4P on HT29 cells is 1.34 µM. It demonstrates that Endostar and CA4P have superimposed cytotoxic effect on HT29 cells. Likewise, K562, and MCF-7 cells remains the same (Figure 1A). The inhibition rate of Endostar alone on six kinds of cells are so extremely low that the inhibition rate is under 30% with the Endostar maximum concentration of 1 mg/mL (Figure 1B). So MNNG/HOS cells and HT29 cells are chosen for further study.
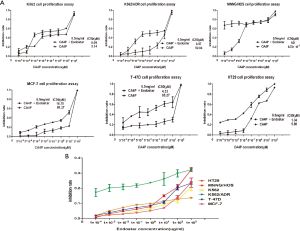
Effect of CA4P and/or Endostar on MNNG/HOS and HT29 cell cycle
CA4P alone induced G2/M phase arrest on MNNG/HOS cells. There was a predominant increase in the blockage at the G2/M phase of MNNG/HOS cell cycle with the concentration of CA4P increasing (Figure 2A). CA4P alone induced G2/M phase arrest on HT29 cells. Endostar alone has no significant G2/M-phase block on HT29 cells. CA4P and Endostar affect percentage of HT29 cells in G2/M phase, although nonsignificant (Figure 2B).
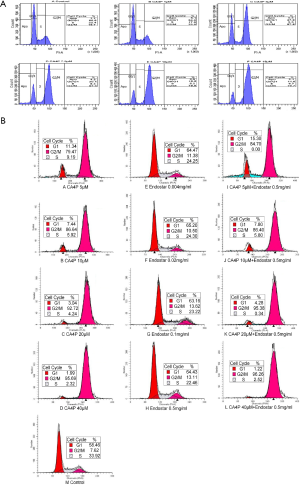
Effect of CA4P and/or Endostar on MNNG/HOS and HT29 cells apoptosis
CA4P alone increased early apoptotic and late apoptotic/necrotic MNNG/HOS cells compared with the controls. Percentages of apoptotic and necrotic MNNG/HOS cells was increased with the concentration of CA4P increasing (Figure 3A). Endostar alone has no significantly increased apoptotic and necrotic effect on HT29 cells. The percentage of apoptosis and necrosis in HT29 cells induced by CA4P and Endostar is higher compared with the CA4P alone (Figure 3B).

Expression of apoptosis-related gene-mRNA
To determine expression of apoptosis-related gene-mRNA on HT29 cell, mRNA levels of caspase 3, caspase 8, caspase 9, Bcl-2, and Bax were detected after exposure to CA4P and/or Endostar. mRNA levels of caspase 3, caspase 8, caspase 9 and Bax were increased in HT29 cells exposed to CA4P alone compared with the (untreated) controls. Besides, caspase 3, caspase 8, caspase 9 and Bax mRNA expression following exposure to CA4P and Endostar was up-regulated significantly compared with the treatment of CA4P alone. Although CA4P and/or Endostar down-regulated expressions of Bcl-2, only the treatment of CA4P and Endostar remained statistically significant (Figure 4).
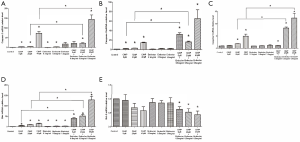
Discussion
Our results indicated that the combination of Endostar and CA4P has the effect of synergistic cytotoxicity and is not limited to anti-angiogenic effects. In the present study, the cell growth inhibition was evaluated under the effects of CA4P and/or Endostar, using six kinds of cells, namely, K562, K562/ADR, MNNG/HOS, MCF-7, T-47D, HT29 cells.
In general, the IC50 values obtained with the combination of CA4P and Endostar were lower than IC50 values obtained with CA4P alone. MTT assay data have shown that the cells could be classified into two groups according to the response to the combination of the two drugs. Group one, including MNNG/HOS, K562/ADR, and T-47D cells, shows that combined Endostar and CA4P have a synergistic effect compared with the CA4P alone. Group two, including HT29, K562, and MCF-7 cells, shows that Endostar in combination of CA4P only have an additional effect compared with the CA4P alone. Results indicate that Endostar exhibited selective cytotoxicity to CA4P cells. Understanding the synergistic cytotoxicity of the combination of Endostar and CA4P on tumor cells is needed (24,25). Exploring the mechanisms of synergistic effect is helpful in guiding the selection of populations that will greatly benefit from the combined treatment of Endostar and CA4P.
Endostar, as a short peptide of collagen XVIII C-terminal, has proliferation inhibition, migration, and apoptotic induction effect on vascular endothelial cells. However, no study explored the effect of Endostar on tumor cells because its mechanisms are extremely complex (25,26). Endostar can widely affect gene expression in vascular endothelial cells and protein phosphorylation according to the study of gene expression profiles. For example, the down-regulation of Bcl-2, Cyclin D, and c-Myc occurs in the phosphorylation of Cyclin B. By contrast, the cytotoxicity of CA4P is strong, and its anti-tumor mechanism is evident. As previously stated, CA4P, as a microtubule-targeting drug, can accelerate microtubule depolymerization. The mechanism of all microtubule-targeting drugs is the disruption of microtubule functions, which can cause mitotic catastrophe and cell apoptosis. Microtubule-targeting drugs hinders sister chromatid exchange by activating a spindle assembly checkpoint during the procedure of abnormal mitosis. The degradation of Cyclin B is then suppressed by promoting compound at the anaphase stage of mitosis APC/C. The high concentration of Cyclin B protein stops the M phase and blocks mitosis. The up-regulation of the expression of myeloid cell leukemia sequence 1 (Mcl-1) protein from the Bcl-2 family can protect cells from apoptosis and provide more time for fixing the wrong assembly of chromosomes (27). The fate of cells depends on randomized competitive result of Cyclin B protein and Mcl-1 protein without external interference (27-30). Whether the cells continue staying in the M phase or apoptosis depends on the degradation speed and relative amounts of Cyclin B and Mcl-1 protein when the wrong assembly of chromosome becomes irreparable. Cells either die in mitosis via apoptosis, or exit mitosis via replication slippage and survive, at which the concentration of Cyclin B protein is lower than the threshold (27). Endogenetic apoptosis, which results in mitotic catastrophe, occurs when Mcl-1 protein fails to inhibit the apoptosis threshold of Bak and Bax (27).
The underlying mechanisms of synergistic effect of Endostar and CA4P are poorly understood. On the basis of the above analysis, Endostar was speculated to change the balance of randomized competitive model during mitosis. It may render cells more susceptible to apoptosis. First, Endostar may cause synergistic effect with CA4P during endogenetic apoptosis and directly influence CA4P to induce cell apoptosis. Second, Endostar may disturb the function of Cyclin B during the upstream of mitotic catastrophe, known as the block of mitosis. Finally, Endostar may arrest cell cycle progression in the M phase and increase the risk of apoptosis.
Conclusions
In summary, the combination of Endostar and CA4P appears hopeful and synergistic cytotoxicity in vitro study, although relative mechanisms need further study. This combined approach may provide a useful insight into exploration that can enhance the effect Endostar in combination with other traditional microtubule-targeting drugs, such as Paclitaxel and vinblastinum. Moreover, Endostar can be new approach for overcoming drug resistance induced by microtubule-targeting drugs and reducing treatment dose.
Acknowledgments
The authors acknowledge the Professor Yong Zou of School of Pharmaceutical Sciences, Sun Yat-sen University.
Funding: This work was supported by the grant from the National Natural Science Foundation of China (No. 81301890), the National Natural Science Foundation of China (No. 81402365) and the Zhejiang Provincial Natural Science Foundation of China (No. LQ17H160012).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.19). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Patterson DM, Rustin GJ. Vascular damaging agents. Clin Oncol (R Coll Radiol) 2007;19:443-56. [Crossref] [PubMed]
- Pettit GR, Singh SB, Niven ML, et al. Isolation, structure, and synthesis of combretastatins A-1 and B-1, potent new inhibitors of microtubule assembly, derived from Combretum caffrum. J Nat Prod 1987;50:119-31. [Crossref] [PubMed]
- Tozer GM, Prise VE, Wilson J, et al. Combretastatin A-4 Phosphate as a tumour vascular-targeting agent: early effects in tumours and normal tissues. Cancer Res 1999;59:1626-34. [PubMed]
- Su J, Laursen BE, Eskildsen-Helmond Y, et al. The vascular-disrupting agent, combretastatin-A4-phosphate, enhances neurogenic vasoconstriction in rat small arteries. Eur J Pharmacol 2012;695:104-11. [Crossref] [PubMed]
- Dark GG, Hill SA, Prise VE, et al. Pettit and Dai J. Chaplin. Combretastatin A-4 agent that displays potent and selective toxicity toward tumor vasculature. Cancer Res 1997;57:1829-34. [PubMed]
- Shen Y, Wu L, Qiu L. Water-Soluble Combretastatin A4 Phosphate Orally Delivered via Composite Nanoparticles With Improved Inhibition Effect Toward S180 Tumors. J Pharm Sci 2017;106:3076-83. [Crossref] [PubMed]
- Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer 2005;5:423-35. [Crossref] [PubMed]
- Li J, Cona MM, Chen F, et al. Sequential systemic administrations of combretastatin A4 Phosphate and radioiodinated hypericin exert synergistic targeted theranostic effects with prolonged survival on SCID mice carrying bifocal tumor xenografts. Theranostics 2013;3:127-37. [Crossref] [PubMed]
- Venegas B, Zhu W, Haloupek NB, et al. Cholesterol Superlattice Modulates CA4P Release from Liposomes and CA4P Cytotoxicity on Mammary Cancer Cells. Biophys J 2012;102:2086-94. [Crossref] [PubMed]
- Garon EB, Neidhart JD, Gabrail NY, et al. A randomized Phase II trial of the tumor vascular disrupting agent CA4P (fosbretabulin tromethamine) with carboplatin, paclitaxel, and bevacizumab in advanced nonsquamous non-small-cell lung cancer. Onco Targets Ther 2016;9:7275-83. [Crossref] [PubMed]
- Garon EB, Kabbinavar FF, Neidhart JA, et al. Randomized phase II trial of a tumor vascular disrupting agent fosbretabulin tromethamine (CA4P) with carboplatin (C), paclitaxel (P), and bevacizumab (B) in stage IIIb/IV nonsquamous non-small cell lung cancer (NSCLC): The FALCON trial. J Clin Oncol 2010;28:7587. [Crossref]
- Zweifel M, Jayson GC, Reed NS, et al. Phase II trial of combretastatin A4 phosphate, carboplatin, and paclitaxel in patients with platinum-resistant ovarian cancer. Ann Oncol 2011;22:2036-41. [Crossref] [PubMed]
- Siemann DW, Chaplin DJ, Walicke PA. A review and update of the current status of the vasculature-disabling agentcombretastatin-A4 phosphate (CA4P). Expert Opin Investig Drugs 2009;18:189-97. [Crossref] [PubMed]
- Siemann DW, Shi W. Dual targeting of tumor vasculature: combining Avastin and vascular disruptingagents (CA4P or OXi4503). Anticancer Res 2008;28:2027-31. [PubMed]
- Fu XH, Li J, Zou Y, et al. Endostar enhances the antineoplastic effects of combretastatin A4 phosphate in an osteosarcoma xenograft. Cancer Lett 2011;312:109-16. [Crossref] [PubMed]
- Versleijen-Jonkers YM, Vlenterie M, van de Luijtgaarden AC, et al. Anti-angiogenic therapy, a new player in the field of sarcoma treatment. Crit Rev Oncol Hematol 2014;91:172-85. [Crossref] [PubMed]
- Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med 1995;333:1757-63. [Crossref] [PubMed]
- Du Y, Zhang Q, Jing L, et al. GX1-conjugated poly (lactic acid) nanoparticles encapsulating Endostar for improved in vivo anticolorectal cancer treatment. Int J Nanomedicine 2015;10:3791-802. [Crossref] [PubMed]
- O'Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997;88:277-85. [Crossref] [PubMed]
- Li Y, Huang XE, Yan PW, et al. Efficacy and safety of Endostar combined with chemotherapy in patients with advanced solid tumors. Asian Pac J Cancer Prev 2010;11:1119-23. [PubMed]
- Zhou JF, Bai CM, Wang YZ, et al. Endostar combined with chemotherapy for treatment of metastatic colorectal and gastric cancer: A pilot study. Chin Med J (Engl) 2011;124:4299-303. [PubMed]
- Zhang Q, Du Y, Jing L, et al. Infra Red Dye and Endostar Loaded Poly Lactic Acid Nano Particles as a Novel Theragnostic Nanomedicine for Breast Cancer. J Biomed Nanotechnol 2016;12:491-502. [Crossref] [PubMed]
- Chen W, Hu S. Suitable carriers for encapsulation and distribution of Endostar: comparison of Endostar-loaded particulate carriers. Int J Nanomedicine 2011;6:1535-41. [PubMed]
- Chen Y, Wang S, Lu X, et al. Cholesterol sequestration by nystatin enhances the uptake and activity of endostatin in endothelium via regulating distinct endocytic pathways. Blood 2011;117:6392-403. [Crossref] [PubMed]
- Zhuo W, Chen Y, Song X, et al. Endostatin specifically targets both tumor blood vessels and lymphatic vessels. Front Med 2011;5:336-40. [Crossref] [PubMed]
- Seppinen L, Pihlajaniemi T. The multiple functions of collagen XVIII in development and disease. Matrix Biol 2011;30:83-92. [Crossref] [PubMed]
- Matson DR, Stukenberg PT. Spindle poisons and cell fate: a tale of two pathways. Mol Interv 2011;11:141-50. [Crossref] [PubMed]
- Rossio V, Galati E, Piatti S. Adapt or die: how eukaryotic cells respond to prolonged activation of the spindle assembly checkpoint. Biochem Soc Trans 2010;38:1645-9. [Crossref] [PubMed]
- Millman SE, Pagano M. MCL1 meets its end during mitotic arrest. EMBO Rep 2011;12:384-5. [Crossref] [PubMed]
- Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell 2008;14:111-22. [Crossref] [PubMed]


