
Combined computed tomography-measured tumor density and RECIST for evaluating neoadjuvant therapy in locally advanced gastrointestinal stromal tumors
Introduction
Gastrointestinal stromal tumors (GISTs) account for only 1–3% of all malignant GI tumors (1). Curative resection is recommended in most cases, with exception for locally advanced gastrointestinal stromal tumors (LAGISTs) which are initially diagnosed as unsuitable for radical resection (2). Neoadjuvant imatinib therapy may be initially indicated if a tumor is borderline resectable or if resection may lead to prominent organ dysfunction. Imatinib can markedly repress GIST progression by inhibiting KIT and PDGFRA tyrosine kinases (2,3). Neoadjuvant imatinib therapy can facilitate surgical resection, reduce tumor spill or bleeding during surgery, or both; however, its role beyond these functions is unclear (4). One previous study observed KIT gene mutations in approximately 80% of GISTs (5). Another study found that primary imatinib resistance occurred more frequently in GISTs with KIT exon 9 mutations, PDGFRA D842V mutations, or wild-type KIT and PDGFRA (6).
GISTs are characterized by abundant hypervascularity (7), and their current gold standard evaluation method is fluorodeoxyglucose positron emission tomography (FDG PET) (8,9). Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) guidelines are also routinely used to evaluate the solid tumor response by using contrast-enhanced computed tomography (CT) (10,11). The most appropriate timing for resection is when shrinkage in tumor size or a decrease in angiogenesis is observed. This will subsequently enhance surgical success, reduce postoperative risks (bleeding/rupture) and complications, and improve the chance of function-sparing surgery. Currently, no well-established clinical tools are available to determine the most appropriate timing for surgical resection of LAGISTs. In this study, we combined CT-measured tumor density and RECIST criteria to evaluate the clinical responses of 12 patients with LAGISTs who received neoadjuvant imatinib therapy.
Methods
Patient demographics
This study recruited 12 consecutive patients diagnosed with LAGISTs who had received neoadjuvant imatinib therapy primarily at the study institution between December 2010 and December 2017. These patients were followed up after neoadjuvant therapy administration for a median of 27 months (range, 11–69 months). In the present study, case series analysis was performed using a routinely updated and maintained electronic medical record database. Demographic data included age at diagnosis, sex, tumor size at presentation (pre- and post-treatment), and origin of the primary tumor. The primary tumor location was categorized into the following regions: the stomach (N=7), rectum (N=2), omentum (N=1), pelvic area (N=1) and pancreas (N=1). All aspects of this study were approved by the institutional review board of Kaohsiung Medical University Hospital (KMUHIRB-G (II)-20160019). This study is a retrospective review of 12 GIST patients and all patients had signed written informed consents.
Pathological and clinical data
All patients’ medical records were reviewed, and their clinicopathological data were obtained through independent re-evaluation of original histopathological slides by two investigators with relevant experience. Both investigators were blinded to the routine diagnoses and patient outcomes. Discrepancies were resolved by simultaneous re-examination of the slides by both investigators. The maximum tumor diameter and mitotic count per 50 high-power fields (HPFs) were determined using the CT or pathological findings for the specimen of each LAGIST case; these examined data were then used to evaluate the risk of death and metastasis (12). The risk of tumor-related death or metastasis was determined by tumor location, tumor size, and mitotic count (per 50 HPFs) (12-18).
Treatment
Each enrolled patient was prescribed imatinib at a dosage of 400 mg/day over a treatment period of 5–30 months. For patients with grade 3 and 4 toxicities, the dosage of imatinib was reduced to 300 mg/day. Sunitinib at a dosage of 37.5 mg/day was applied as second-line therapy after first-line neoadjuvant imatinib therapy failed and disease progression was shown on computed tomography images. Regorafenib at a dosage of 120 mg/day was used as third-line treatment.
Evaluation of tumor response and toxicities
The tumor size and density of these enrolled patients were confirmed after their abdominal CT images were examined by two radiologists. Discrepancies were resolved by simultaneous re-examination of the images by both radiologists. Tumor responses were evaluated using oncologic findings and CT images showing changes in tumor density in addition to applying RECIST 1.1 (8,10). The CT attenuation coefficient (density) was categorized as follows: grade 4, >30% decrease; grade 3, 11–30% decrease; grade 2, ≤10% decrease or increase; and grade 1, >10% increase (8).
In this study, we proposed that combining CT-measured tumor density and RECIST may be useful to determine the timing of surgical resection for patients with LAGISTs; this combined method suggests either a reduction of more than 30% for tumor density, or a tumor size reduction of more than 30% for a partial response (PR), as determined using RECIST 1.1. A complete response was defined as the disappearance of all lesions; PR was defined as a tumor size reduction of 30% or more or a tumor density reduction of 30% or more on CT images. In addition, stable disease (SD) was defined as having no symptomatic deterioration attributed to tumor progression, and progression disease (PD) was defined as an increase in tumor size of 20% or more and not meeting the criteria of PR based on tumor density on CT images. Adverse events (AE) were graded according to the Common Terminology Criteria for Adverse Events, version 3.0 (http://ctep.cancer.gov/reporting/ctc.html).
KIT and PDGFRA gene mutation analysis
To obtain cancer tissue samples, CT-guided core biopsies were performed before neoadjuvant therapy administration. The biopsy specimens were embedded in paraffin, fixed in formalin, and then serially cut into 4-µm thick sections. DNA extraction was performed using the QIAGEN DNA extraction kit (QIAGEN Inc., Valencia, USA), according to the manufacturer’s protocol. The concentration and quality of the extracted DNA were then determined using the NanoDrop 2000 spectrophotometer. The optical density at 260 or 280 nm of DNA derived from all patient specimens ranged from 1.8 to 2.0; thus, DNA samples were of high quality and suitable for subsequent experiments. The DNA samples were analyzed using polymerase chain reaction (PCR) performed on a PCR instrument from Applied Biosystems (Thermo Fisher Scientific Inc., MA, USA). Next, the KIT or PDGFRA primer (100 µM) and the 2X Tag polymerase reaction mix were added.
Statistical analyses
All statistical analyses were performed using SPSS version 19.0 (SPSS, Inc., Chicago, IL, USA). Progression-free survival (PFS) was measured from the start date of treatment to the date of any type of progression or the final follow-up. Overall survival (OS) was defined as the period from the start of treatment to death from any cause or the final follow-up. The OS and PFS were plotted using the Kaplan-Meier method.
Results
Follow-up duration and survival evaluation
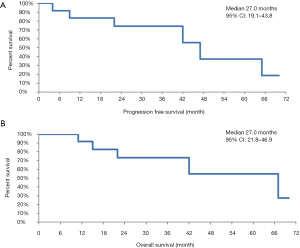
The median PFS was 27 months (range, 4–69 months) (Figure 1A) and the median OS was 27 months (range, 11–69 months) (Figure 1B). Among the 12 patients, 7 were still alive at the final follow-up conducted in December 2017.
Patient series, tumor characteristics, and mutation status
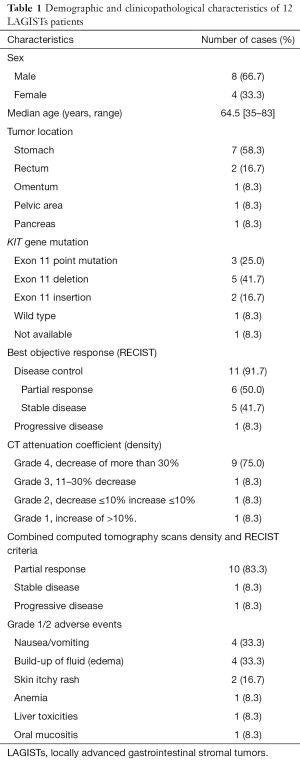
Twelve patients with pathologically confirmed GISTs were identified during the study period. Table 1 provides a summary of their demographic and clinicopathological characteristics. The median age was 64.5 years (range, 35–83 years), and there were 8 (66.7%) male and 4 (33.3%) female patients. LAGISTs were located in the stomach of 7 patients (58.3%), the rectum of 2 patients (16.7%), the omentum of 1 patient (8.3%), the pelvic area of 1 patient (8.3%) and pancreas of 1 patient (8.3%).
Full table
KIT and PDGFRA gene mutation analysis revealed KIT exon 11 deletions in 5 patients (41.7%), KIT exon 11 insertion in 2 patients (16.7%), and KIT exon 11 point mutations in 3 patients (25.0%). Wild-type KIT and PDGFRA were noted in 1 patient (8.3%). The genotype of 1 patient (8.3%) was not sequenced due to insufficient specimen material.
Although RECIST 1.1 is the most well-established and widely used evaluation approach, its treatment efficacy for GIST is limited. Hence, we also applied CT-measured tumor density for better evaluation. The best clinical responses of 6 patients (50.0%) were PR evaluated with RECIST 1.1. Using our combined CT-measured tumor density and RECIST 1.1 for response evaluation, the best clinical responses of 10 patients (83.3%) were considered as PR. One other patient (8.3%) was a borderline PR with a tumor density reduction of 28% but was considered as SD using our combined evaluation approach (case 6).
Notably, 9 patients (75%) who were simultaneously evaluated using RECIST 1.1 combined with CT-measured tumor density had more than 30% decrease in CT-measured tumor density (Table 1). No grade 3 or 4 AEs were observed during neoadjuvant imatinib therapy, and the two most common AEs (grade 1 or 2) were nausea/vomiting and edema (33.3% for both, Table 1).
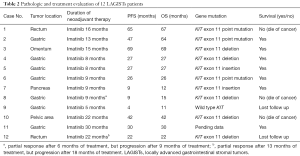
The pathologic and treatment evaluation of each patient is presented in Table 2. Table 3 shows the treatment outcome of all 12 patients. For all patients, their maximum tumor diameter before treatment ranged from 5.9 to 15.4 cm. We found that neoadjuvant imatinib therapy might decrease the tumor risk of GIST by reducing both tumor size and mitotic count (Table 3). Six patients (case 1, 2, 3, 4, 5, 7) with optimal tumor volume shrinkage showed PR evaluated using our combined CT tumor density and RECIST 1.1 approach had undergone radical resection (R0 resection) after 8–16 months of neoadjuvant imatinib therapy. One other patient (case 6) who was a borderline PR with a tumor density reduction of 28% but was considered as SD using our combined evaluation approach also had radical resection (R0 resection) (Table 3).
Full table
Full table
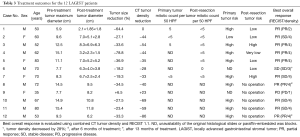
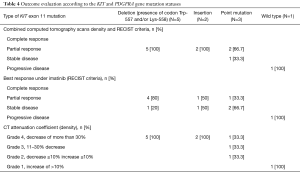
Additionally, mutations in KIT exon 11 were observed in ten patients. Using our combined CT-measured tumor density and RECIST 1.1 method, all 5 patients (100%) with deletions of Trp-557, Lys-558, or both codons showed a PR and were categorized as grade 4 based on the CT attenuation coefficient (density). Using only RECIST 1.1 to evaluate the 5 patients with deletions in KIT exon 11, four patients (80%) showed a PR and one patient (20%) showed SD (Table 4).
Full table
When assessed using our combined evaluation approach, two patients with an insertion in KIT exon 11 were considered to have a PR (100%). Two (66.7%) of 3 patients with point mutations were considered to show a PR and 1 patient (33.3%) showed SD. By contrast, when patients with point mutations were assessed using only RECIST 1.1, 1 patient (33.3%) showed a PR and 2 patients (66.7%) showed SD. Each of these 3 patients with point mutations was characterized as grade 4 (33.3%), grade 3 (33.3%), and grade 2 (33.3%) according to the CT attenuation coefficient (density) (Table 4).
Discussion
The standard treatment for primary GISTs is complete surgical resection. However, for some cases, complete resection is either unsuitable or impossible because of the anatomic site, tumor size, and high risk of rupture or metastasis (2). Given the hypervascularity of GISTs, the risk of rupture and eventual seeding or bleeding can be significant and lead to poor prognosis (9). Hence, determining the optimal treatment duration and the most appropriate timing for resection is critical in patients requiring potential resection. Currently, FDG PET is globally recognized as the gold standard evaluation method for GISTs. However, its high cost limits its use in routine imaging studies, and it is not feasible in patients whose baseline FDG PET results are negative for tumors (8).
Instead, contrast-enhanced CT is routinely used to evaluate the solid tumor response according to RECIST 1.1. Although RECIST 1.1 is a more favorable indicator of treatment response, the major drawback of RECIST 1.1 is that it relies solely on tumor size to monitor treatment response (8). By contrast, the criteria of Choi et al. exhibits high sensitivity and specificity in responders as it evaluates treatment response by assessing changes in the size (a tumor size reduction of more than 10%) or density of the target lesions (a tumor density reduction of more than 15% on CT images) (8,19). However, a minor alteration in tumor size or vascularity may have limited significance when evaluating the benefits of surgery in patients with LAGISTs.
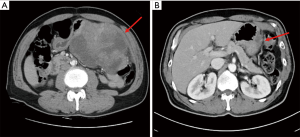
Thus, our combined CT-measured tumor density and RECIST 1.1 method may be beneficial for deciding an appropriate timing of surgical resection in LAGISTs. This combined method suggests either a significant reduction of more than 30% for tumor density or a tumor size reduction of more than 30% defines a PR and thus suggest LAGIST patients could be resected. Case 4 was one of the examples that had both a significant reduction of more than 30% for tumor density and a tumor size reduction of more than 30% (Figure 2).
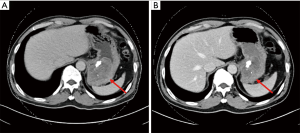
We discovered that 3 (42.9%) of the 7 patients who underwent resection had significant decrease in tumor density despite being determined as SD according to RECIST 1.1. Although neither patient exhibited a remarkable decrease in tumor size, surgical bleeding was reduced and radical resection was also achieved. For instance, CT tumor density of case 6 had a significant reduction of nearly 30% and had subsequently undergone radical resection (Figure 3). This finding may be attributed to the decrease in tumor density, which corresponded to inhibited angiogenesis.
During the first 6 months of treatment, the treatment outcome was a PR in case 8, but disease progression was observed after the subsequent 3 months of treatment (Table 3). According to the gene mutation profile, only case 9 exhibited wild-type KIT and PDGFRA, and disease progressed after 4 months of treatment then lost follow-up; case 9 also showed disease progression identified by RECIST 1.1, which was consistent with the CT-measured tumor density finding. Moreover, although cases 10 and 11 showed SD as determined using RECIST 1.1, a notable decrease in CT-measured tumor density was observed; thus, these cases were considered PRs when evaluated using our combined CT tumor density and RECIST 1.1 method. Case 12 was considered to have a PR after 13 months of treatment, but disease progressed after 18 months of treatment.
Based on data from established clinical studies, imatinib is approved as a first-line neoadjuvant therapy for LAGISTs and as an adjuvant therapy without defining a specific treatment duration for patients with intermediate- to high-risk primary GISTs after resection (20,21). Neoadjuvant imatinib therapy can enhance operative potential and reduce surgical complications and recurrence (22). Imatinib can markedly repress GIST activity by inhibiting the KIT and PDGFRA tyrosine kinases (3). One previous study reported that approximately 80% of GISTs exhibited KIT gene mutations (5), and our current study is consistent with this prior finding as KIT gene mutations were found in 10 of 11 patients (90.9%).
Various types of KIT exon 11 mutations have been discovered to be prognostically significant (23). A marked poorer disease-free survival and clinical outcome were observed in GIST patients with KIT exon 11 insertions or deletions (including Trp-557, Lys-558, or both codons) than in those with other types of exon 11 mutations or wild-type KIT (24,25). Despite that, KIT exon 11 mutation was not a useful predictor of tumor response and resectability in our study.
Among the 10 patients with KIT exon 11 mutations, two patients exhibited insertion mutations and were considered to have a PR when evaluated using our combined CT tumor density and RECIST 1.1 evaluation approach. The CT attenuation coefficient (density) for both patients was grade 4. Both patients in our study did not have a remarkably poorer disease-free survival and clinical outcome than those with other types of exon 11 mutations or wild-type KIT.
Five patients who exhibited deletions in Trp-557, Lys-558, or both codons were considered to have a PR when evaluated using our combined CT-measured density and RECIST 1.1 method. Moreover, all five showed a CT-measured tumor density reduction of more than 30%. When RECIST 1.1 guideline was used, four of the five patients (80%) showed a PR and one (20%) showed SD (Table 4).
Three patients with point mutations had contradictory results when evaluated using RECIST 1.1 and our combined evaluation approach. Determined using our combined CT tumor density and RECIST 1.1 evaluation method, 2 of 3 patients with KIT point mutations were considered to have PR and 1 patient showed SD, whereas 1 patient showed a PR and 2 patients showed stable disease (SD) according to RECIST 1.1 (Table 4).
Additionally, primary imatinib resistance occurs more frequently in GISTs with wild-type KIT and PDGFRA (6). Consistent with the finding of Lee et al. (6), the only case with wild-type KIT and PDGFRA in our study eventually showed disease progression. For patients who do not respond to imatinib treatment, determining when to switch the regimen is equally crucial. Therefore, precise objective evaluation of the treatment response influences the regimen plan and is critical to patients with GISTs.
This study highlights the importance of utilizing both CT-measured tumor density and RECIST 1.1 for plausible surgical resection timing consideration. Comparing RECIST 1.1, CT-measured tumor density, and KIT and PDGFRA gene mutation profiles, our results suggest that our combined CT-measured tumor density and RECIST 1.1 method is more suitable for evaluating surgical resectability in patients with LAGISTs. However, a rather small sample size and a median follow-up duration of 27 months may be the limitations of this study. Future investigations could include a larger sample size and lengthening follow-up duration. We argue that long-term follow-up is crucial for LAGIST management, along with the aforementioned evaluation approach to determine tumor response and feasible surgical resection timing.
Conclusions
Our combined CT-measured tumor density and RECIST 1.1 method may potentially offer an alternative evaluation approach that may be beneficial for deciding the preferable timing for surgical resection of LAGISTs.
Acknowledgments
Funding: This work was supported by grants from the Kaohsiung Medical University Hospital (KMUH104-4M25, KMUH104-4M51, KMUHHU105-5M21, KMUH106-6R32, KMUH106-6M28, KMUH106-6M29, KMUH106-6M30, KMUH106-6M31, KMUHS10522, KMUHS10505, KMUHS10518, and KMUHGCRC2016002, KMUHS10601,KMUHS10608, KMUHA10664). In addition, this study was supported by Kaohsiung Medical University “Aim for the Top 500 Universities Grant” (KMU-TP105C01, KMU-TP105C11) Kaohsiung, Taiwan; “Aim for the Top University Grant,” under grant nos. KMU-S105011, KMU-TP105A14, KMU-DK106005, and SH000113 (Give2Asia); and the Grant of Biosignature in Colorectal Cancers, Academia Sinica, Taiwan.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.34). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All aspects of this study were approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-G (II)-20160019). This study is a retrospective review of 12 GIST patients and all patients had signed written informed consents.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gastrointestinal Stromal Tumors - NORD (National Organization for Rare Disorders) [Internet]. NORD (National Organization for Rare Disorders). 2014 [cited 29 January 2018]. Available online: https://rarediseases.org/rare-diseases/gastrointestinal-stromal-tumors/
- Coffey RJ, Washington MK, Corless CL, et al. Ménétrier disease and gastrointestinal stromal tumors: hyperproliferative disorders of the stomach. J Clin Invest. 2007;117:70-80. [Crossref] [PubMed]
- Casali PG, Jost L, Reichardt PESMO Guidelines Working Group, et al. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20:64-7. [PubMed]
- Wang D, Zhang Q, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of radiation therapy oncology group 0132. Ann Surg Oncol 2012;19:1074-80. [Crossref] [PubMed]
- Joensuu H. Gastrointestinal stromal tumor (GIST). Ann Oncol 2006;17:x280-6. [Crossref] [PubMed]
- Lee JH, Kim Y, Choi JW, et al. Correlation of imatinib resistance with the mutational status of KIT and PDGFRA genes in gastrointestinal stromal tumors: a meta-analysis. J Gastrointestin Liver Dis 2013;22:413-8. [PubMed]
- Cavallaro G, Polistena A, D'Ermo G, et al. Duodenal gastrointestinal stromal tumors: review on clinical and surgical aspects. Int J Surg 2012;10:463-5. [Crossref] [PubMed]
- Choi H, Charnsangavej C, de Castro Faria S, et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol 2004;183:1619-28. [Crossref] [PubMed]
- Gelmini R, Bertolini F, Rossi G, et al. Laparoscopic approach of gastric gastrointestinal stromal tumors (GISTs):is it still a courageous choice? Report of two cases. Surg Laparosc Endosc Percutan Tech 2007;17:133-7. [Crossref] [PubMed]
- Tirkes T, Hollar MA, Tann M, et al. Response criteria in oncologic imaging:review of traditional and new criteria. Radiographics 2013;33:1323-41. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors:A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Miettinen M, Sobin LH. Gastrointestinal stromal tumors in the appendix: a clinicopathologic and immunohistochemical study of four cases. Am J Surg Pathol 2001;25:1433-7. [Crossref] [PubMed]
- Miettinen M, Kopczynski J, Makhlouf HR, et al. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol 2003;27:625-41. [Crossref] [PubMed]
- Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach:a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 2005;29:52-68. [Crossref] [PubMed]
- Miettinen M, Makhlouf H, Sobin LH, et al. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol 2006;30:477-89. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [Crossref] [PubMed]
- Kim YJ, Kim SS. Gastrointestinal Stromal Tumor. Korean J Helicobacter Up Gastrointest Res 2011;11:82-9. [Crossref]
- Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 2007;25:1753-9. [Crossref] [PubMed]
- von Mehren M, Randall RL, Benjamin RS, et al. Soft Tissue Sarcoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:758-86. [Crossref] [PubMed]
- Blay JY, von Mehren M, Blackstein ME. Perspective on updated treatment guidelines for patients with gastrointestinal stromal tumors. Cancer 2010;116:5126-37. [Crossref] [PubMed]
- Seshadri RA, Rajendranath R. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors. J Cancer Res Ther 2009;5:267-71. [Crossref] [PubMed]
- Andersson J, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors with KIT exon 11 deletions are associated with poor prognosis. Gastroenterology 2006;130:1573-81. [Crossref] [PubMed]
- Wardelmann E, Losen I, Hans V, et al. Deletion of Trp-557 and Lys-558 in the juxtamembrane domain of the c-kit protooncogene is associated with metastatic behavior of gastrointestinal stromal tumors. Int J Cancer 2003;106:887-95. [Crossref] [PubMed]
- Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 2008;112:608-15. [Crossref] [PubMed]


