Vessel-centered laparoscopic total mesorectal excision via medial approach
Introduction
Total mesorectal excision (TME) is currently the standard treatment for rectal cancer. This procedure provides significant oncological benefits by reducing local recurrence and increasing 5-year survival. Laparoscopic surgery for colorectal cancer has been steadily developing since its introduction in 1990, and laparoscopic TME (lap TME) is an ideal minimally invasive surgical technique for rectal cancer. Compared with laparotomy, laparoscopic surgery has similar safety, completeness of resection, and prognosis (1,2).
The primary principle of rectal TME is to find an anatomical plane to ensure the integrity of visceral layer of the rectum during the surgery. Therefore, laparoscopic lateral and medial approaches are developed based on the membrane anatomy. Experienced surgeons may adopt the lateral approach to reach the correct plane; however, entering the posterior renal space by mistake is common. At present, the medial approach is more widely used; that is, the dissection starts at the level of iliac crest or the root of the mesenteric artery and then travels upwards along the abdominal aorta to cut open the sigmoid mesocolon and thus find an appropriate anatomical plane; subsequently, the root of mesenteric blood vessel is skeletonized to remove lymph nodes. Another principle of the radical surgery for tumors is to prioritize the handling of blood vessels, including dissection of perivascular lymphatic adipose tissue and transection of blood vessels, during which the veins are transected first, followed by the transection of arteries, so as to lower the risk of tumor metastasis via the blood stream during the surgery. During TME, the inferior mesenteric artery (IMA) and its accompanying vessels should be skeletonized, and the inferior mesenteric vein (IMV) should be transected first. In addition, under the premise of ensuring the radical treatment of tumors, the nourishing vessels (e.g., the left colonic artery) of the anastomosis of the intestine should be carefully protected. An increasing number of evidences have shown that the transection of the left colonic vessels may increase the risk of postoperative anastomotic leak. TME via the conventional laparoscopic medial approach typically follows the order of layer-vessel, which may result in the dissociation of mesangium due to early separation of the Toldt’s space behind the mesorectum, the exposure of left colonic blood vessels and IMV, and difficulty in the dissection of lymph nodes at the root of IMA. Therefore, when following the principles of TME, we altered the order of layer-vessel in conventional medial approach based on the need for vessel handling and lymph node dissection in rectal cancer patients and proposed the concept of handling vessels before expanding layers; that is, the vascular pathway is dissected first, and then the posterior rectal layer is extended, which is known as the vessel-centered laparoscopic TME via medial approach. The advantages of this procedure include: (I) it completely follows the principles of radical treatment of tumors, minimizing the risk of tumor metastasis via blood stream; (II) compared with the layer-vessel practice, the vessels are relatively fixed since the posterior vascular space has not been mobilized, and dissection along the blood vessels can easily expose the IMA trunk, left colonic vessels, and IMV; (III) the full exposure of the blood vessels is conducive to the dissection of inter-vascular lymph nodes, especially the No. 253 lymph node; (IV) it is conducive to the preservation of left colonic vessels; and (V) it simplifies the surgical process and shortens the operation. The specific operation steps of this procedure are as follows.
Indications and contraindications
Indications
Preoperative rectal MRI and/or intrarectal endoscopic ultrasonography reveal clinical cT1-cT3 stage and N-negative rectal cancer or lower sigmoid colon cancer. Preoperative rectal MRI and/or intrarectal endoscopic ultrasonography reveals suspiciously positive circumferential resection margin (CRM) and/or N-positive middle to lower rectal cancer that is ≤12 cm away from the anal margin. Neoadjuvant radiochemotherapy followed by surgery is recommended.
Contraindications
Tumor-related contraindications: with positive or suspiciously positive CRM, including severe infiltration of organs around the rectal cancer, massive pelvic tumors, and intestinal obstruction that can not be adequately decompressed; patient-related contraindications: poor systemic conditions; accompanied by other severe diseases; and/or unable to tolerate anesthesia and surgery.
Anesthesia, surgical position, trocar sites, and positions of medical staff
Anesthesia
Tracheal intubation under general anesthesia is performed, and continuous epidural anesthesia may be added.
Surgical position
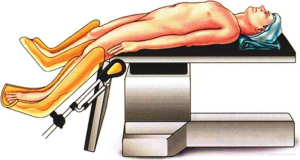
The patient is placed on a lithotripsy position, with right hip joint held at 45° of extension and abduction and knee joint at 30° of flexion. The hips are raised, and the lower limbs are lower than the hips. The right upper limb is adducted, and the left upper limb is adducted or abducted, when necessary. After surgery begins, the position is adjusted to feet higher than head by 30-degree angle, with the body tilted to the right by 15-degree (Figure 1).
Trocar placement
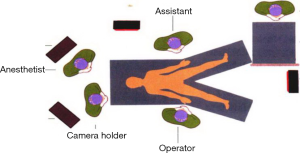
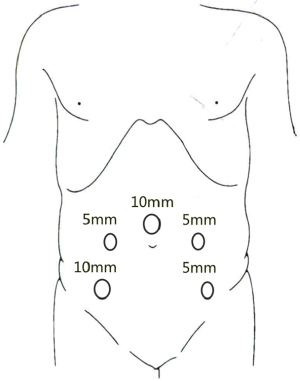
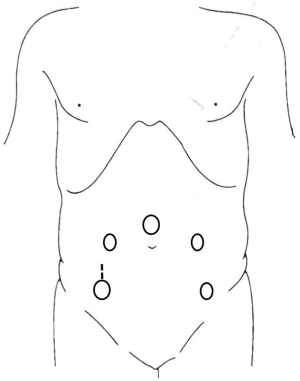
A 10-mm trocar is placed on the upper edge of the umbilicus, and the laparoscope is placed as an observation port after the trocar is inflated. A second 12-mm trocar is placed in the right lower abdomen (intersection between right midclavicular line and the connecting line of bilateral anterior superior iliac spines) under the laparoscope and used as the main operating port. A 5-mm trocar is placed at the left midclavicular line at the level of the umbilicus as the auxiliary operating port. A second 5-mm trocar is placed at intersection between left midclavicular line and the connecting line of bilateral anterior superior iliac spines as the auxiliary operating port (Figures 2 and 3).
Surgical steps and key points
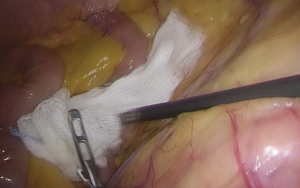
- After the trocars are inserted, the assistant moves to the left side of the patient, above the operator. The patient’s position is adjusted feet higher than head by 30-degree angle and tilted by 15–20 degrees. This allows the small intestine to move to the upper abdominal cavity, exposing the root of the small-bowel mesentery (Figure 4). Gauze is used to fend off the small intestine (Figure 5).
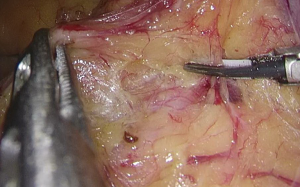
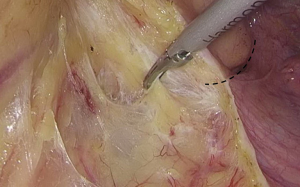
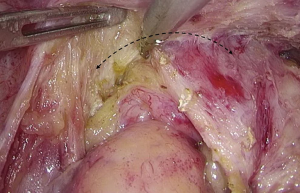
- The sigmoid colon and rectum are pulled leftwards and upwards to expose the right mesentery of the rectum, creating a groove-like space between the inferior mesenteric vascular pedicle and the posterior peritoneum. The posterior peritoneum is cut open along this line, up to the lower edge of the mesenteric attachment of the small bowel and down to the sacrum (Figures 6 and 7
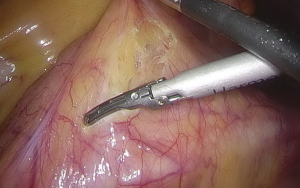
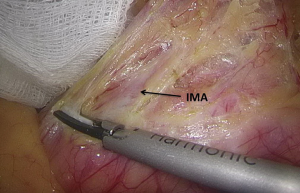
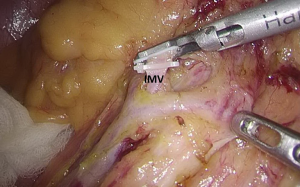
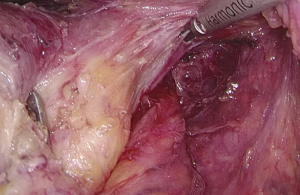
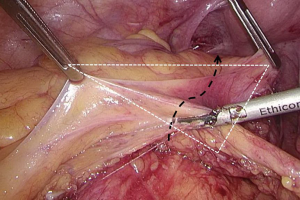
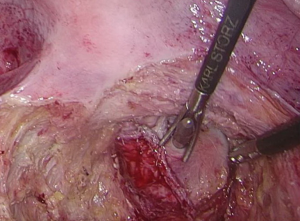
). - After the peritoneum in front of the abdominal aorta is cut open, raise the peritoneum to separate adipose tissue until the white sheath of IMA becomes visible. After the traveling of IMA is confirmed, the adipose and lymphoid tissues are dissociated to the distal end along the vascular sheath (Figures 8-11
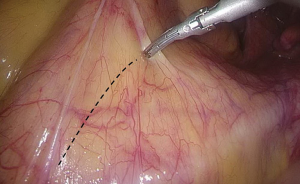
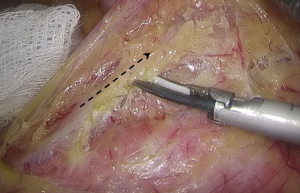
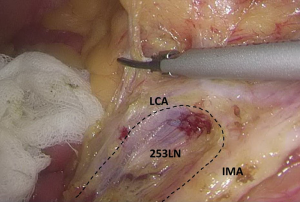
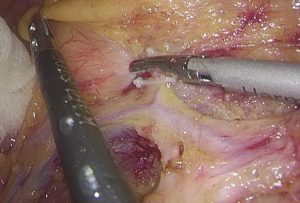
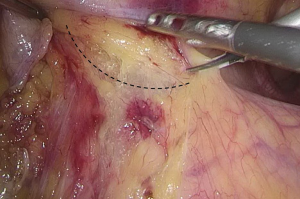
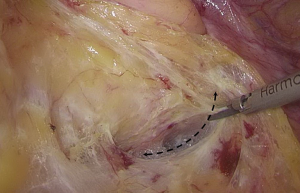
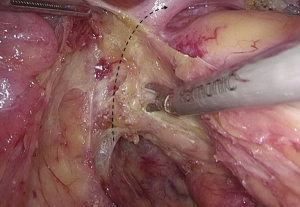
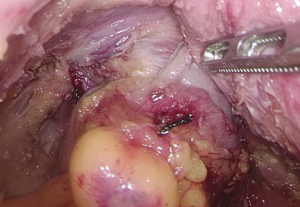
). - Separation toward the distal portion till the site where the IMA gives off the left colic artery (LCA), which travels towards the left upper proximal end. Dissection towards the proximal end continues along the LCA, during which No. 253 lymph node that exists among LCA, IMA, and abdominal aorta is removed (Figures 12-15
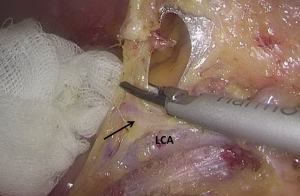
). 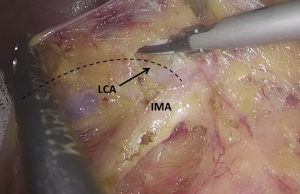 Figure 12 The curved mark is the traveling of the left colic artery (LCA) (indicated by dotted line). IMA, inferior mesenteric artery.
Figure 12 The curved mark is the traveling of the left colic artery (LCA) (indicated by dotted line). IMA, inferior mesenteric artery.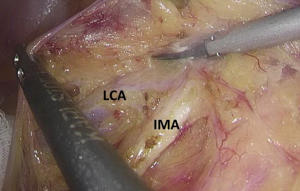 Figure 13 Dissociation of vessels along LCA. LCA, left colic artery; IMA, inferior mesenteric artery.
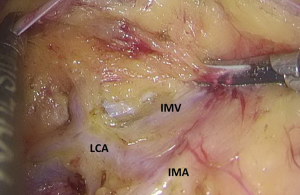
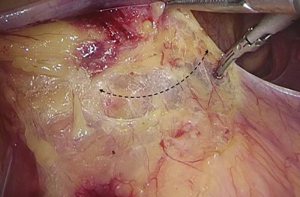
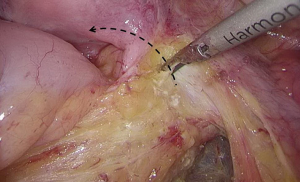
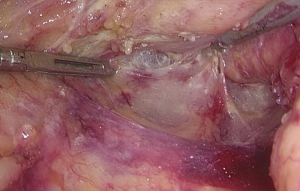
Figure 13 Dissociation of vessels along LCA. LCA, left colic artery; IMA, inferior mesenteric artery. - Dissection continues towards the distal end along the LCA and IMA planes. Typically, the IMV travels along the lower or lateral side of LCA. Dissection along this plane allows the dissociation of superior rectal artery (Figures 16 and 17
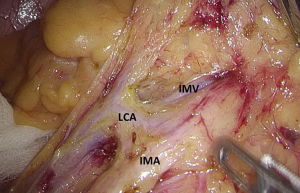
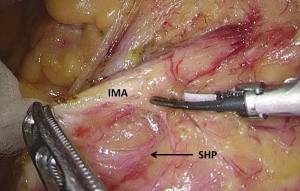
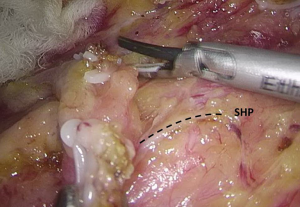
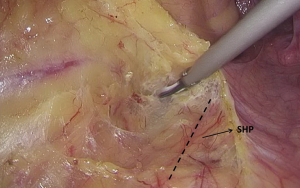
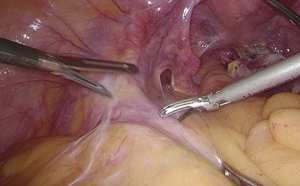
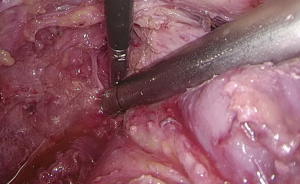
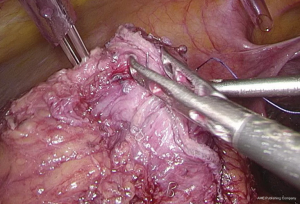
). - The space behind the IMA is isolated, during which the scalpel should be close to the vessel and the superior hypogastric plexus (SHP), left ureter, and left reproductive vessels should be carefully protected (Figures 18 and 19
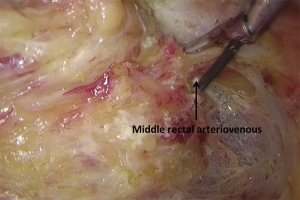
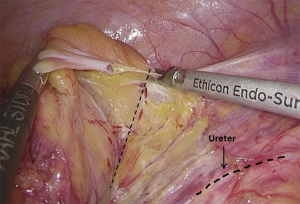
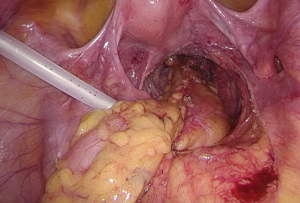
). - Transection of inferior mesenteric vein and superior rectal artery: when the Toldt’s space behind the rectum can be clearly seen and the left ureter and genital vessels become visible, dissection continues laterally till the left lateral paracolic gutter, during which the superior hypogastric plexus should be protected (Figures 20-23
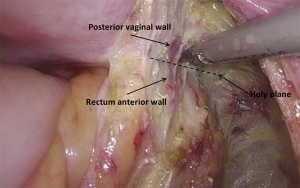
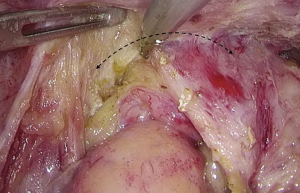
). - Dissection of the retrorectal space: the retrorectal space, also known as Holy space, is an avascular plane between visceral fascia and parietal fascia. It contains a small amount of loose connective tissue and autonomic nerve that governs the pelvic organs, with the sacrococcygeal ligament as its lower boundary and the lateral ligament as the lateral side. The root of the transected blood vessel is raised and the rectum is pulled forwards and towards the head side. After the sharp dissection and separation between visceral fascia and parietal fascia of mesorectum, the retrorectal space (between the hypogastric nerve and the intrinsic fascia of the rectum) can be precisely entered (Figures 24-26Figures 27-31
). - Separation of lateral rectal space: open the lateral peritoneum along the yellow-white junction and extend the lateral peritoneum incision. The assistant pulls the rectum toward the head side and the reverse side. The good traction between the operator and the assistant enables the maintenance of adequate tension. Attention should be paid to protect the lateral pelvic nerves (Figures 32-35
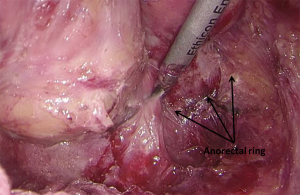
). - Separation of the superior space of levator ani: separate downwards the peritoneal reflection till the level of retrorectal space; when there is a sense of resistance, it means the rectosacral fascia has been reached. At this point, the dense sacrorectal fascia needs to be cut open, which allows the entering of a loose space (the superior space of levator ani). Dissection continues towards the anal side till the vertical plane of the levator ani. After the presacral space is entered and isolated, pampiniform presacral venous plexus is visible, which should be carefully protected. The posterior and lateral sides of the rectum are separated till the edge of the hiatus of levator anus muscle, which its landmark being the puborectalis that surrounds the rectum. Then, the puborectalis is thoroughly mobilized (Figures 36 and 37
). - Isolation of prerectal space: peritoneum is usually cut open along a curved line 1.0 cm above the peritoneal reflection and isolated in front of Denonvilliers’ fascia. In males, the Denonvilliers’ fascia is cut open at the bottom of seminal vesicle. In females, since there is no obvious anatomic landmark, a full-thickness dissection of Denonvilliers’ fascia performed near the distal mesorectum (Figures 38 and 39
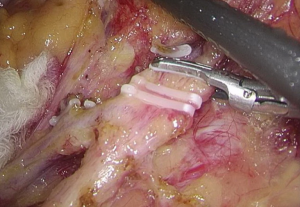
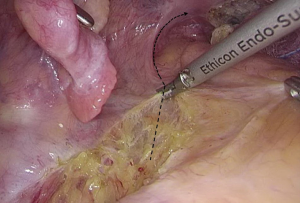
). - Rectal transection: the lower edge of the tumor can be identified by rectal examination and then marked with a titanium clip. The distal resection margin is approximately 2–3 cm above the lower edge of the tumor. Carefully isolate the mesorectum on the lateral and posterior walls of rectum and avoid any injury or penetration of the intestinal wall. After anal irrigation, a rotatable cutter-stapler is placed through the main operating trocar. The angle of the cutter-stapler is adjusted after the intestinal wall is clamped. The assistant standing at the distal side assists in completely placing the intestine into the cutting range of the cutter-stapler by using a pair of noninvasive pliers. The bowl is then stapled; typically, two closures are required, during which there must be an overlapping area between these two closures to ensure complete closure of the stump (Figures 40 and 41
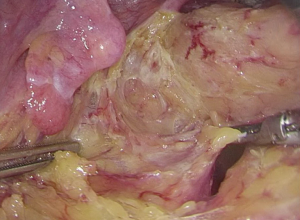
). - Mobilize and clip the mesorectum to be resected: lift the rectal stump and dissociate it towards the proximal side along the Toldt’s line. After the sigmoid and parts of the descending colon are thoroughly mobilized, lift the distal and proximal portions of the rectum and the transected rectal vascular pedicles to form a triangular plane (as shown in the figure). Mobilize the mesorectum along the pre-cut line (Figures 42 and 43
). - Harvest the specimen: typically, a 4–5-cm incision is made under the umbilicus. For patients requiring preventive colostomy, the colostomy incision (i.e., the right main operating port) can be fully utilized. The incision can be enlarged and appropriately extended, via which the specimen is mobilized. The intestinal canal is transected about 10 cm away from the proximal side of the tumor to remove the specimen (Figures 44-47
). - Rectal anastomosis: a 29–33-mm stapler is inserted after the anus is dilated. The puncture site should be at the intersection of two closures to minimize the risk of postoperative anastomotic leakage. Check the presence of any bowel volvulus before stapling. An air leakage test may be performed after the stapling. After the abdominal and pelvic cavities are rinsed with dilute iodophor, a dual cannula is placed at the low-posterior portion of the rectum (Figures 48-50
). If the serous surface of the tumor is involved (as judged during the operation) or intraperitoneal implantation is highly possible, 2–3 doses of raltitrexed (3 mg/m2) were routinely administered after rinsing. The drug is dissolved in 200 mL of normal saline for intraperitoneal chemotherapy. The drainage tube is clamped for 1–2 h before reopening.
Comments
Rectal TME is recognized as the “gold standard” in the radical treatment of rectal cancer and can effectively reduce the local recurrence of rectal cancer. It emphasizes the following principles: (I) to dissect along the roots of the tumor draining blood vessels to maximize lymph node removal; and (II) to find and maintain the surgical plane based on human embryology and anatomy to ensure that the visceral fascia is smooth and complete, without defects. Laparoscopic TME also needs to follow the basic principles of open TME and meanwhile has higher requirements on the knowledge of the anatomy of organs and tissues around the rectum.
First, the scope of blood vessel handling and lymphatic dissection must be decided. There are two ways to handle the IMA during rectal TME: one is high ligation, which refers to ligation at the start of the IMA from the abdominal aorta; and the other is low ligation, which refers to the ligation at the start of the left colonic artery. During the laparoscopic TME, surgeons tend to mobilize the IMA and perform high ligation at its root. By doing so, they can simply and effectively dissect deep lymph nodes and quickly find correct anatomical spaces for TME (3,4). However, simplicity is not the only justification for a surgical procedure; rather, the following two factors should also be considered: (I) the necessity of deep lymph node dissection; and (II) the need for the preservation of left colonic vessels.
Lymph nodes at the root of IMA are the station 3 nodes of rectal cancer. Two independent studies have demonstrated the correlation between the metastasis to station 3 nodes and the tumor T stage. The rates of metastasis to station 3 nodes were 0%, 0.4%, 2.6%, and 2.9% for stages T1, T2, T3, and T4 tumors in study A (5) and 0%, 1.0%, 2.6%, and 4.3% in study B (6). The corresponding data in Fudan University Cancer Hospital were 0%, 0.95%, 5.22%, and 6.12%, suggesting the degree of lymph node metastasis along the IMA is associated with the local infiltration of tumors, and the metastasis to station 3 nodes mainly occurs in T3 and T4 tumors. In addition, station 3 is the only affected station in about 6% of the rectal cancer patients, which is known as the skipped metastasis to station 3 nodes (7). Therefore, for T1 and T2 rectal cancers, theoretically low ligation is sufficient to achieve the radical resection of the tumors. However, the problem is that there is currently no way to accurately assess preoperative and intraoperative tumor T stage to guide the resection range of lymph nodes (8), not to mention the possibility of skipped metastasis and micrometastasis. Therefore, it is highly necessary to dissect the station 3 nodes during the surgical treatment of resectable rectal cancer.
After high ligation of the IMA, the blood supply to the descending colon and sigmoid colon entirely comes from the mid-colon artery and the marginal arterial arch (9,10). While some studies suggest that the blood supply from the mid-coital artery and the peripheral arterial arch is sufficient to maintain the survival of distal colon and anastomosis (11), most studies have demonstrated that high ligation of the IMA significantly reduces blood supply to the distal colon and anastomosis (12,13). Seike et al. found in their intraoperative measurements that approximately one-fifth of patients experienced significant reduction in anastomotic blood supply after high ligation, especially among elderly males and in patients who had received lower anterior resection, and preservation of the left colonic artery could alleviate this condition (14). We had the same finding by using indocyanine green angiography: the blood flow at the proximal colonic anastomosis and the marginal arterial arch was significantly reduced after blocking the left colonic vessels. In addition, because of the absence of collateral circulation between superior mesenteric artery and IMA in some patients, severe anastomotic ischemia may occur after surgery, which may further lead to anastomotic stricture and anastomotic leak (15). Therefore, preservation of the left colonic vessels during surgery will help reduce the incidence of anastomotic leak, especially in elderly males or in patients with low anastomosis.
After the weighing of pros and cons, surgeons have developed a modified surgical procedure for rectal cancer: the IMA is ligated at the site below the origin of the LCA, followed by the dissection of adipose and lymphatic tissues (No. 253 lymph node) between the LCA and the IMA (5). It has been confirmed that the number of lymph nodes collected during LCA-preserving station 3 lymph node dissections was not significantly different from the number of lymph nodes harvested during high ligation (16). Notably, compared with high ligation, laparoscopic LCA-preserving D3 radical resection requires longer operative time (17). The development and popularization of this procedure require higher surgical skills and a simpler and more reasonable surgical approach.
A lateral approach and a medial approach have been applied in laparoscopic TME. The lateral approach is adopted from open surgery; however, it is seldom used because the operator may mistakenly operate on the posterior renal space during surgery. The medial approach is more commonly applied. In 2004, the European Association for Endoscopic surgery (EAES) formally identified it as the recommended approach for laparoscopic colorectal resection (18). The classic medial approach is as follows: first, enter the Toldt’s space from the midline side (i.e., behind the IMA) to expose the nerve plexus, ureter, and reproductive system vessels behind the mesentery; second, mobilize and expand this level to the congenital adhesion of the lateral peritoneum; third, mobilize and ligate the root of the blood vessel; fourth, mobilize the mesentery and dissect lymph nodes; and finally, mobilize all the intestinal parts. As a plane-vessel technique, this procedure has many advantages including fully exposed surgical field, clear anatomical plane, and less blood loss. The length of the resected intestine, the length of the tumor’s inferior resection margin, and the number of lymph nodes removed are comparable to those in the open surgery group. However, left colic blood vessel-preserving D3 dissection remains a challenging task in clinical settings. For example, early isolation of the Toldt’s space behind the rectum may cause the mesangial detachment and the distortion of the local anatomic landmarks, which may result in difficulty in exposing the LCA; as a result, it is impossible to preserve the left colic vessels and carry out effective dissection of No. 253 lymph node.
Following the principle of TME and based on the needs for lymphatic dissection, we changed the surgical sequence from plane-vessel to vessel-plane; that, the vascular path is dissected first, and the plane at the retrorectal space is extend when handling the vessels. This is the concept of vessel-centered laparoscopic TME via medial approach. First, the retroperitoneum is opened with an ultrasonic scalpel at the sigmoid mesocolon and retroperitoneal reflection; however, instead of entering and expanding the Toldt’s space at this time, the peritoneum is opened at the projecting root of the IMA and the inferior mesenteric arterial sheath is exposed. Dissection along the vascular sheaths continues till the root of LCA is exposed. The surgical plane is expanded upwards and downwards along the root of LCA and IMA. Since the posterior vascular space has not been mobilized, the local anatomical plane is relatively fixed; thus, it is relatively easy to expose the left colic vessels, No. 253 lymph node, IMV, superior rectal vessels, and sigmoid branch vessels. After vascular skeletonization, the No. 253 lymph node was removed, followed by the successive transection of IMV and superior rectal artery. After the above operations were completed, the assistant lifts the stumps of distal mesangial vessels, and the Toldt’s space behind the rectum can be clearly visible. The remaining TME operation steps are completed along this space.
Compared with the traditional medial approach, this procedure has the following advantages: (I) the blood vessels are handled first, which is in line with the principles of oncology and can minimize the risk of bloodborne metastasis; (II) since the vessels are relatively fixed since the posterior vascular space has not been mobilized, and dissection along the blood vessels can easily expose the IMA trunk, left colonic vessels, and IMV; (III) the exposure of blood vessels in the drainage area is conducive to the dissection of the adipose and lymphatic tissues, especially the No. 253 lymph node; (IV) the preferential exposure of the left colic vessels can increase the probability of preserving these blood vessels and help reduce the incidence of anastomotic leakage after operation; (V) dissection and transection of blood vessels, dissection of lymph nodes, and exposure of the posterior space are all performed in front of the posterior subperitoneal fascia, thus obviously reducing the risk of injuring the retroperitoneal vessels, ureters, and autonomic nerves; and (VI) it simplifies the surgical process, and the whole process of exposure and dissection requires only 10–15 minutes.
In summary, with the use of high-definition and 3D camera systems, precise dissection and skeletonization of blood vessels can be better performed, which increases the accuracy of surgeries. As a result, a reasonable approach and dissection sequence have become increasingly important for high-quality TME. The vessel-centered laparoscopic TME via the medial approach, as a simplified and safe procedure, is more consistent with the principles of radical treatment for tumors and deserves further application in clinical settings.
About the author
Xinxiang Li (Figure 51), male, born in 1971, chief physician, professor, doctoral tutor, and deputy director of the Department of Colorectal Surgery, Cancer Hospital Affiliated to Fudan University. He is also the head of the Endoscopic Surgery Panel of the Professional Committee of Minimally Invasive Treatment for Cancer of Shanghai Anti-cancer Society, vice chairman of Shanghai Municipal Professional Committee of Pelvic Disease, member of the CSCO Expert Committee on Colorectal Cancer, vice chairman of the Subcommittee of Laparoscopy of the Colorectal Cancer Committee of the Chinese Medical Doctor Association, member of the Chinese Association of Colorectal Surgeons, member of the Chinese Association of Endocrinologists, member of the standing committee and deputy secretary-general of the Professional Committee of Cancer Surgery of the Chinese Research Hospital Association, member of the standing committee of the Professional Committee of Oncology of the Chinese Research Hospital Association, and member of the Laparoscopy Panel of the Professional Committee of Colorectal Cancer of the Chinese Anticancer Association.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.44). The authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210-8. [Crossref] [PubMed]
- Kennedy RH, Francis EA, Wharton R, et al. Multicenter randomized controlled trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme: EnROL. J Clin Oncol 2014;32:1804-11. [Crossref] [PubMed]
- Hartley JE, Mehigan BJ, Qureshi AE, et al. Total mesorectal excision: assessment of the laparoscopic approach. Dis Colon Rectum 2001;44:315-21. [Crossref] [PubMed]
- Morino M, Parini U, Giraudo G, et al. Laparoscopic total mesorectal excision: a consecutive series of 100 patients. Ann Surg 2003;237:335-42. [Crossref] [PubMed]
- Pandey D. Survival benefit of high ligation of the inferior mesenteric artery in sigmoid colon or rectal cancer surgery (Br J Surg 2006; 93: 609-615). Br J Surg 2006;93:1023-author reply 1023. [Crossref] [PubMed]
- Chin CC, Yeh CY, Tang R, et al. The oncologic benefit of high ligation of the inferior mesenteric artery in the surgical treatment of rectal or sigmoid colon cancer. Int J Colorectal Dis 2008;23:783-8. [Crossref] [PubMed]
- Kim JC, Lee KH, Yu CS, et al. The clinicopathological significance of inferior mesenteric lymph node metastasis in colorectal cancer. Eur J Surg Oncol 2004;30:271-9. [Crossref] [PubMed]
- Wald C, Scheirey CD, Tran TM, et al. An update on imaging of colorectal cancer. Surg Clin North Am 2006;86:819-47. [Crossref] [PubMed]
- Hida J, Yasutomi M, Maruyama T, et al. Indication for using high ligation of the inferior mesenteric artery in rectal cancer surgery. Examination of nodal metastases by the clearing method. Dis Colon Rectum 1998;41:984-7; discussion 987-91. [Crossref] [PubMed]
- Liang JT, Huang KC, Lai HS, et al. Oncologic results of laparoscopic D3 lymphadenectomy for male sigmoid and upper rectal cancer with clinically positive lymph nodes. Ann Surg Oncol 2007;14:1980-90. [Crossref] [PubMed]
- Goligher JC. The adequacy of the marginal blood-supply to the left colon after high ligation of the inferior mesenteric artery during excision of the rectum. Br J Surg 1954;41:351-3. [Crossref] [PubMed]
- Dworkin MJ, Allen-Mersh TG. Effect of inferior mesenteric artery ligation on blood flow in the marginal artery-dependent sigmoid colon. J Am Coll Surg 1996;183:357-60. [PubMed]
- Corder AP, Karanjia ND, Williams JD, et al. Flush aortic tie versus selective preservation of the ascending left colic artery in low anterior resection for rectal carcinoma. Br J Surg 1992;79:680-2. [Crossref] [PubMed]
- Seike K, Koda K, Saito N, et al. Laser Doppler assessment of the influence of division at the root of the inferior mesenteric artery on anastomotic blood flow in rectosigmoid cancer surgery. Int J Colorectal Dis 2007;22:689-97. [Crossref] [PubMed]
- Siddharth P, Smith NL. An anatomic basis to prevent ischemia of the colon during operations upon the aorta. Surg Gynecol Obstet 1981;153:71-3. [PubMed]
- Sekimoto M, Takemasa I, Mizushima T, et al. Laparoscopic lymph node dissection around the inferior mesenteric artery with preservation of the left colic artery. Surg Endosc 2011;25:861-6. [Crossref] [PubMed]
- Kobayashi M, Okamoto K, Namikawa T, et al. Laparoscopic lymph node dissection around the inferior mesenteric artery for cancer in the lower sigmoid colon and rectum: is D3 lymph node dissection with preservation of the left colic artery feasible? Surg Endosc 2006;20:563-9. [Crossref] [PubMed]
- Veldkamp R, Gholghesaei M, Bonjer HJ, et al. Laparoscopic resection of colon Cancer: consensus of the European Association of Endoscopic Surgery (EAES). Surg Endosc 2004;18:1163-85. [Crossref] [PubMed]