
Surgical stress biomarkers as early predictors of postoperative infectious complications in patients with colorectal cancer
Introduction
Colorectal cancer (CRC) is considered one of leading culprits of cancer-related mortality that is also associated with frequent morbidity and is the third most common cancer in China (1,2). Although surgical advancements have reduced the postoperative mortality over the last few decades, the postoperative morbidity has remained high (3-6). In addition to the morbidity that patients suffer from, severe postoperative complications negatively affect postoperative oncological outcomes, quality of life, financial burden and long-term prognosis (7-10). In detail, CRC patients who undergo resection have a high incidence of infectious complications, which can increase recurrence rates (11,12). The ability to predict the risk of complications before treatment would most likely facilitate the early detection and management of complications after surgery.
It has been shown that surgical trauma can cause systemic inflammatory responses (13,14). Furthermore, surgical stress induces the release of various cytokines, resulting in postoperative morbidity, such as fever and pain, as well as infectious complications and anastomotic leakage (15-17). Tumor necrosis factor-α (TNF-α) and interleukin-10 (IL-10) have been considered important biomarkers for surgical stress (18,19). In detail, TNF-α is proinflammatory and induces an acute phase reaction with local and systemic inflammation, while IL-10 acts as an anti-inflammatory interleukin that inhibits the synthesis of proinflammatory cytokines and shifts the immune response from Th1 type to Th2 type (20-22). It has been shown that TNF-α and IL-10 could predict the occurrence of postoperative complications in patients who underwent pancreatectomy (19). However, there was insufficient data concerning the correlation of serum TNF-α and IL-10 levels with postoperative infectious complications associated with colorectal surgery.
In the present study, we evaluated and compared the significance of perioperative serum TNF-α and IL-10 levels as early predictors of infectious complications after colorectal surgery.
Methods
Study population
Data from 200 primary CRC patients who underwent curative resection via an open approach at the Third Xiangya Hospital of Central South University between July 1, 2012, and July 1, 2017, were retrospectively collected in a planned database. The inclusion criteria were as follows: histologically confirmed stage I, II or III CRC; age older than 18 years; life expectancy of more than 24 weeks; and complete data in terms of TNF-α and IL-10 levels and hospital course. Patients with acute and severe infections or sepsis, other malignancies and autoimmune disorders at the time of surgery and those who received preoperative adjuvant chemotherapy were excluded from the study. Open colorectal surgery was performed through a vertical midline incision or a transrectal incision of minimal length. Bowel anastomosis was performed by a double-stapling technique. All patients were administered perioperative antimicrobial prophylaxis. This study was approved by the Institutional Review Board of the Third Xiangya Hospital (Ethical approval ID, 20170823).
Clinical assessment
The demographic and clinicopathological data of all patients, including gender, age, tumor site, BMI, TNM stage and surgical time and blood loss, were collected from the clinical records by one surgeon and checked by another surgeon. Clinical stage was evaluated by postoperative tumor tissue histopathological examination and clinical assessment according to the UICC TNM classification (23).
Data on infectious complications within 30 days of surgery, including anastomotic leakage, intraabdominal abscesses, surgical site infections (SSIs), central line infections and other organ system infections, were collected and evaluated by the attending surgeon based on patient data and medical records. Anastomotic leakage was diagnosed based on the properties of the drainage fluid or radiographic findings. Abscesses were confirmed by purulent drainage fluid or during relaparotomy. The diagnosis of SSI was based on clear signs of inflammation at the surgical wound or purulent drainage from the wound. A central line infection was verified though positive blood cultures and cultures from the catheter tip. Other organ system infections were confirmed by radiographic studies and laboratory tests based on the respective diagnostic standards.
Evaluation of serum TNF-α and IL-10 levels
A total of 5 mL of EDTA-treated blood samples were obtained on preoperative day 1 and postoperative day (POD) 0, 1, 3 and 5 from all subjects. These samples were then centrifuged at 3000 rpm for 10 minutes and then kept at −80 °C until later analyses. Serum levels of TNF-α and IL-10 were evaluated with a commercially available sandwich enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions (R&D systems, Minneapolis, MN, USA).
Statistical analysis
Statistical analysis of the data was performed with SPSS 20.0 (IBM SPSS, Armonk, New York, USA). P <0.05 (two sided) was considered statistically significant. Continuous variables are represented by the mean ± SD, and categorical variables are represented by frequencies. The χ2 test or Fisher’s exact test was used to compare categorical variables, while continuous variables were analyzed by independent Student’s t-test. The cut-off values for serum TNF-α and IL-10 levels for predicting infectious complications were calculated by a ROC curve analysis. The logistic Cox regression model was used to assess the OR and multivariate analysis.
Results
Patient characteristics
In our analysis of 200 CRC patients, the median age was 58 years (range, 53–72 years), and 43.2% of patients were female. Eighty-seven (43.5%) patients were diagnosed with colon cancer, whereas 113 (56.5%) patients had rectal cancer. There were 65 (32.5%) patients identified as at an early tumor stage (stage I or II) and 135 (67.5%) patients with advanced cancer (stage III), according to the TNM staging protocol.
A total of 27 (13.5%) patients experienced postoperative infectious complications. Among these patients, 23 (11.5%) patients had minor complications, while 4 (2.0%) patients suffered from major complications. The most common infectious complication was anastomotic leakage (n=13, 5.5%), followed by SSIs (n=9, 3.5%) and other organ system infections (n=5, 1.5%). Among patients with other organ system infections, 2 patients had pneumonia and 3 patients experienced urinary tract infections (UTIs). No cases of central line infections or intraabdominal abscesses were reported. There were several clinical characteristics associated to occurrence of infectious, including BMI, surgical time, blood loss, perioperative IL-10 and TNF-α levels, see Table 1. Patients with pneumonia and UTIs were treated with antibiotics. SSIs mainly affected the median incision without dehiscence of the fascia and were treated with drainage and antibiotics. Among patients with anastomotic leakage, 2, 4 and 7 patients were treated with antibiotics alone, an interventional drainage procedure and relaparotomy, respectively.
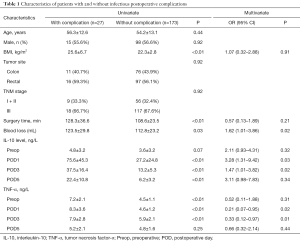
Full table
Correlation between IL-10 levels and infectious complications
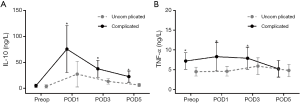
The serum levels of IL-10 on PODs 1, 3 and 5 were markedly different among patients who had complications compared with patients who had an uneventful postoperative course (Figure 1). In detail, for patients with complications, the mean IL-10 levels on PODs 1, 3 and 5 were 75.6±45.3, 37.5±16.4 and 22.4±10.8 ng/L, respectively, compared with 27.2±24.8, 13.2±5.3 and 6.2±3.2 ng/L, respectively, in those patients without complications (all P<0.05). However, there was no significant difference in the mean preoperative IL-10 level between patients with complications and those without complications (4.8±3.2 vs. 3.6±3.2 ng/L, P=0.07). In multivariate analysis, mean IL-10 level on POD 1 (OR, 3.28; 95% CI: 1.31–9.42; P=0.03) and POD 3 (OR, 1.47; 95% CI: 1.01–3.82; P=0.02) were independently related to occurrence of infectious complication (Table 1).
Furthermore, postoperative IL-10 levels were also higher in patients who developed anastomotic leakage than in those without leakage. In particular, among patients who had anastomotic leakage, the IL-10 levels on PODs 1, 3 and 5 were 74.3±26.8, 45.8±18.4 and 23.9±5.2 ng/L, respectively, vs. 35.8±23.8, 28.2±12.3 and 14.2±6.3 ng/L, respectively, for those patients who did not have anastomotic leakage (all P<0.05; Table 2).
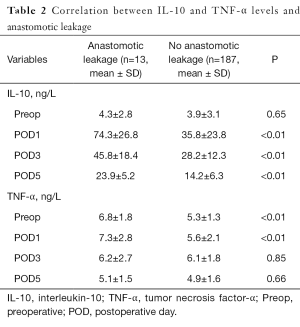
Full table
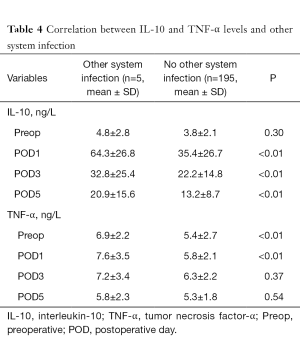
For patients with SSIs, the initial postoperative increase in IL-10 was higher than that in uncomplicated SSI cases (all P<0.05; Table 3). IL-10 peaked on POD 1, with a mean IL-10 level of 84.5±52.8 ng/L, and did not show a marked decrease during the following days. In patients with other organ system infections, the postoperative IL-10 level was also higher than in those who had an uneventful course (all P<0.05; Table 4).
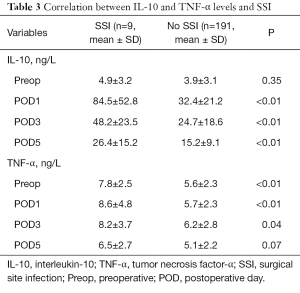
Full table
Full table
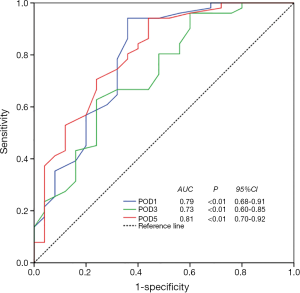
The results of the ROC curve analysis that assessed the cut-off value for serum IL-10 to predict postoperative infectious complications are shown in Figure 2. A serum IL-10 level >72.4 ng/L on POD 1 was associated with the occurrence of infectious complications, with a sensitivity of 94.1% and a specificity of 64.0% (AUC: 0.79, P<0.01, 95% CI: 0.68–0.91, Figure 2). Similarly, a serum IL-10 level >35.3 ng/L on POD 3 was associated with the occurrence of infectious complications, with a sensitivity of 62.7% and a specificity of 76.0% (AUC: 0.73, P<0.01, 95% CI: 0.60–0.85, Figure 2). Finally, the cutoff value for IL-10 level on POD 5 for predicting infectious complications was 20.3 ng/L, with a sensitivity of 94.1% and a specificity of 56.0% (AUC: 0.81, P<0.01, 95% CI: 0.70–0.92, Figure 2).
Correlation between TNF-α levels and infectious complications
As with IL-10, the TNF-α levels on preoperative day 1 and PODs 1 and 3 were significantly different between patients who experienced infectious complications and those who had an uneventful course (Figure 1B). In patients who had infectious complications, the TNF-α levels on preoperative day 1 and PODs 1 and 3 were 7.2±2.1, 8.3±3.6 and 7.9±2.8 ng/L, respectively, vs. 4.5±1.1, 4.6±1.2 and 5.9±2.1 ng/L, respectively, in patients who had an uneventful course (all P<0.05). On POD 5, the TNF-α levels of patients with complications and those without complications were not markedly different (5.2±2.1 vs. 4.8±1.6 ng/L, P=0.25). In multivariate analysis, we found that mean TNF-α level on POD 1 (OR, 0.21; 95% CI: 0.07–0.95; P=0.02) and POD 3 (OR, 0.33; 95% CI: 0.12–0.97; P=0.01) were independently related to occurrence of infectious complication.
For patients with anastomotic leakage, it was observed that the TNF-α levels of complicated cases on preoperative day 1 and POD 1 were higher than in uncomplicated cases (6.8±1.8 vs. 5.3±1.3 ng/L and 7.3±2.8 vs. 5.6±2.1 ng/L, respectively; all P<0.05, Table 2). However, the TNF-α levels on PODs 3 and 5 were not significantly different between complicated cases and uncomplicated cases (all P>0.05, Table 2). Furthermore, for comparison of patients with other organ system infections and those without complications, the TNF-α levels on preoperative day 1 (6.9±2.2 vs. 5.4±2.7 ng/L, P<0.01), POD 1 (7.6±3.5 vs. 5.8±2.1 ng/L, P<0.01), POD 3 (7.2±3.4 vs. 6.3±2.2 ng/L, P=0.37) and POD 5 (5.8±2.3 vs. 5.3±1.8 ng/L, P=0.54) had similar trends (Table 4).
The TNF-α levels of patients who had SSIs on preoperative day 1 and PODs 1 and 3 were also higher than in those who did not have SSIs. Among patients who had SSIs, the TNF-α levels on preoperative day 1 and PODs 1 and 3 were 7.8±2.5, 8.6±4.8 and 8.2±3.7 ng/L, respectively, compared with 5.6±2.3, 5.7±2.3 and 6.2±2.8 ng/L, respectively, in those without SSIs (all P<0.05, Table 3).
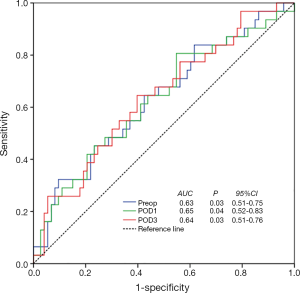
In the ROC curve analysis, a TNF-α level >6.9 ng/L on preoperative day 1, >8.1 ng/L on POD 1 and >7.5 ng/L on POD 3 were associated with the occurrence of infectious complications. The sensitivity was 32.3%, and the specificity was 90.4% for a TNF-α level >6.9 ng/L on preoperative day 1 (AUC: 0.63, P=0.03, 95% CI: 0.51–0.75, Figure 3); the sensitivity was 80.6%, and the specificity was 45.2% for a TNF-α level >8.1 ng/L on POD 1 (AUC: 0.65, P=0.04, 95% CI: 0.52–0.83, Figure 3); and the sensitivity was 64.5%, and the specificity was 60.3% for a TNF-α level >7.5 ng/L on POD 3 (AUC: 0.64, P=0.03, 95% CI: 0.51–0.76, Figure 2).
Discussion
Infectious complications after colorectal surgery are considered one of the most serious and life-threatening clinical problems (24-27). Early detection of infectious complications after surgery is important for initiating effective treatment and improving clinical outcomes (28,29). Biomarkers that predict postoperative infectious complications would be extremely helpful. It is well known that colorectal resection leads to pronounced surgical stress and induces inflammatory and anti-inflammatory responses, resulting in postoperative morbidity and infectious complications (30,31). The existing evidence in the literature suggests that TNF-α and IL-10 are involved in the pathogenesis of postoperative surgical stress (32). In addition, a previous study reported that the peritoneal cytokine levels of TNF-α and IL-10 were additional diagnostic biomarkers that supported the early prediction of anastomotic leakage after colorectal surgery (33). The current study sought to assess if serum TNF-α and IL-10 levels were valuable as early predictors of infectious complications after colorectal surgery.
In present study, we specifically confirmed that serum TNF-α and IL-10 levels were important biomarkers for assessing the risk of postoperative infectious complications in CRC patients. Of note, the incidence of infectious complications in the current study was consistent with the literature. Furthermore, we observed a direct correlation between serum IL-10 levels on PODs 1, 3 and 5 and the risk of experiencing infectious complications. TNF-α was also found to be highly predictive of infectious complications, especially on preoperative day 1 and PODs 1–3. However, the line plots of TNF-α and IL-10 levels during the perioperative period were different in peak points and trends, reflecting the dynamic changes in the inflammatory and anti-inflammatory responses. Therefore, the TNF-α/IL-10 ratio may more accurately reflect surgical stress.
Furthermore, we found that the postoperative IL-10 level tended to significantly higher among patients who developed anastomotic leakage, SSIs or other organ system infections. The TNF-α levels on preoperative day 1 and PODs 1–3 were higher among patients with anastomotic leakage, SSI or other organ system infections. In the ROC analysis, cut-off values were identified to assess the clinical relevance of IL-10 and TNF-α levels. A serum IL-10 level >72.4 ng/L on POD 1, >35.3 ng/L on POD 3 and >20.3 ng/L on POD 5 had high sensitivity and specificity for detecting postoperative infectious complications. A TNF-α level >6.9 ng/L on preoperative day 1, >8.1 ng/L on POD 1 and >7.5 ng/L on POD 3 were confirmed to be sensitive and specific predictors of complications.
There were several limitations in our study. One problem was the retrospective, relatively small scale and single center design of the study. A large scale multicenter prospective study would allow further confirmation of the predictive significance of surgical stress biomarkers in CRC patients. Furthermore, all subjects enrolled in this study were patients who underwent surgery for primary CRC, so the results may not be applicable to those undergoing surgery for benign disease. Finally, the inherent inaccuracy of ELISA assays may have influenced the accuracy of the measurement of serum cytokine levels; thus, flow cytometry may be necessary to obtain more accurate serum cytokine levels to analyze (34).
In conclusion, our study has identified sensitive and specific surgical stress biomarkers that are predictive of clinically relevant infectious complications and may guide clinical practice. Particularly during the early postoperative period, IL-10 and TNF-α were strongly correlated with a higher risk of infectious complications after CRC surgery. The assessment of IL-10 and TNF-α levels may therefore facilitate the management of patients undergoing CRC resection. These results may potentially guide postoperative care, such as modifications in antibiotic therapy, different drain management, and early imaging, to detect and prevent complications.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.40). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Third Xiangya Hospital (No. 20170823). Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Chen W. Cancer statistics: updated cancer burden in China. Chin J Cancer Res 2015;27:1. [PubMed]
- Riedy M. Preventing colorectal cancer. Adv NPs PAs 2013;4:18-21; quiz 2. [PubMed]
- Rybakov EG, Shelygin YA, Khomyakov EA, et al. Risk factors for postoperative ileus after colorectal cancer surgery. Colorectal Dis 2017; [Epub ahead of print]. [PubMed]
- Bayar B, Yilmaz KB, Akinci M, et al. An evaluation of treatment results of emergency versus elective surgery in colorectal cancer patients. Ulus Cerrahi Derg 2015;32:11-7. [Crossref] [PubMed]
- McSorley ST, Khor BY, MacKay GJ, et al. Examination of a CRP first approach for the detection of postoperative complications in patients undergoing surgery for colorectal cancer: A pragmatic study. Medicine (Baltimore) 2017;96:e6133 [Crossref] [PubMed]
- Aoyama T, Oba K, Honda M, et al. Impact of postoperative complications on the colorectal cancer survival and recurrence: analyses of pooled individual patients' data from three large phase III randomized trials. Cancer Med 2017;6:1573-80. [Crossref] [PubMed]
- McSorley ST, Watt DG, Horgan PG, et al. Postoperative Systemic Inflammatory Response, Complication Severity, and Survival Following Surgery for Colorectal Cancer. Ann Surg Oncol 2016;23:2832-40. [Crossref] [PubMed]
- Prenner S, Levitsky J. Comprehensive Review on Colorectal Cancer and Transplant. Am J Transplant 2017;17:2761-74. [Crossref] [PubMed]
- Saraste D, Martling A, Nilsson PJ, et al. Complications after colonoscopy and surgery in a population-based colorectal cancer screening programme. J Med Screen 2016;23:135-40. [Crossref] [PubMed]
- Kvasnovsky CL, Adams K, Sideris M, et al. Elderly patients have more infectious complications following laparoscopic colorectal cancer surgery. Colorectal Dis 2016;18:94-100. [Crossref] [PubMed]
- Moyes LH, Leitch EF, McKee RF, et al. Preoperative systemic inflammation predicts postoperative infectious complications in patients undergoing curative resection for colorectal cancer. Br J Cancer 2009;100:1236-9. [Crossref] [PubMed]
- Cata JP, Velasquez JF, Ramirez MF, et al. Inflammation and pro-resolution inflammation after hepatobiliary surgery. World J Surg Oncol 2017;15:152. [Crossref] [PubMed]
- Lindholm EE, Aune E, Seljeflot I, et al. Biomarkers of inflammation in major vascular surgery: a prospective randomised trial. Acta Anaesthesiol Scand 2015;59:773-87. [Crossref] [PubMed]
- Shibata J, Ishihara S, Tada N, et al. Surgical stress response after colorectal resection: a comparison of robotic, laparoscopic, and open surgery. Tech Coloproctol 2015;19:275-80. [Crossref] [PubMed]
- Andersson B, Ansari D, Norden M, et al. Surgical stress response after colorectal resection. Int Surg 2013;98:292-9. [Crossref] [PubMed]
- Munteanu A, Munteanu D, Tigan S, et al. How do surgical stress and low perioperative serum protein and albumin impact upon short term morbidity and mortality in gastric cancer surgery? Clujul Med 2017;90:71-85. [Crossref] [PubMed]
- Haga Y, Wada Y, Takeuchi H, et al. Evaluation of modified Estimation of Physiologic Ability and Surgical Stress in gastric carcinoma surgery. Gastric Cancer 2012;15:7-14. [Crossref] [PubMed]
- Dimopoulou I, Armaganidis A, Douka E, et al. Tumour necrosis factor-alpha (TNFalpha) and interleukin-10 are crucial mediators in post-operative systemic inflammatory response and determine the occurrence of complications after major abdominal surgery. Cytokine 2007;37:55-61. [Crossref] [PubMed]
- Kvarnstrom AL, Sarbinowski RT, Bengtson JP, et al. Complement activation and interleukin response in major abdominal surgery. Scand J Immunol 2012;75:510-6. [Crossref] [PubMed]
- Pieringer H, Danninger K, Tzaribachev N, et al. Patients with arthritis undergoing surgery: how should we manage tumour necrosis factor blocking agents perioperatively?-A systematic literature review. Yonsei Med J 2013;54:253-7. [Crossref] [PubMed]
- Talwalkar SC, Grennan DM, Gray J, et al. Tumour necrosis factor alpha antagonists and early postoperative complications in patients with inflammatory joint disease undergoing elective orthopaedic surgery. Ann Rheum Dis 2005;64:650-1. [Crossref] [PubMed]
- Tong LL, Gao P, Wang ZN, et al. Can lymph node ratio take the place of pN categories in the UICC/AJCC TNM classification system for colorectal cancer? Ann Surg Oncol 2011;18:2453-60. [Crossref] [PubMed]
- Parthasarathy M, Greensmith M, Bowers D, et al. Risk factors for anastomotic leakage after colorectal resection: a retrospective analysis of 17 518 patients. Colorectal Dis 2017;19:288-98. [Crossref] [PubMed]
- Gessler B, Eriksson O, Angenete E. Diagnosis, treatment, and consequences of anastomotic leakage in colorectal surgery. Int J Colorectal Dis 2017;32:549-56. [Crossref] [PubMed]
- Boyce SA, Harris C, Stevenson A, et al. Management of Low Colorectal Anastomotic Leakage in the Laparoscopic Era: More Than a Decade of Experience. Dis Colon Rectum 2017;60:807-14. [Crossref] [PubMed]
- Chaudhury PK, Jeschke MG, Monson JR, et al. What is the diagnostic value of C-reactive protein for the prediction and the exclusion of postoperative infectious complication after colorectal surgery? Can J Surg 2014;57:417-9. [Crossref] [PubMed]
- Stinner B, Bauhofer A, Lorenz W, et al. Granulocyte-colony stimulating factor in the prevention of postoperative infectious complications and sub-optimal recovery from operation in patients with colorectal cancer and increased preoperative risk (ASA 3 and 4). Protocol of a controlled clinical trial developed by consensus of an international study group. Part three: individual patient, complication algorithm and quality manage. Inflamm Res 2001;50:233-48. [Crossref] [PubMed]
- Welsch T, Muller SA, Ulrich A, et al. C-reactive protein as early predictor for infectious postoperative complications in rectal surgery. Int J Colorectal Dis 2007;22:1499-507. [Crossref] [PubMed]
- Mari G, Costanzi A, Crippa J, et al. Surgical Stress Reduction in Elderly Patients Undergoing Elective Colorectal Laparoscopic Surgery within an ERAS Protocol. Chirurgia (Bucur) 2016;111:476-80. [Crossref] [PubMed]
- Tabuchi T, Shimazaki J, Satani T, et al. The perioperative granulocyte/lymphocyte ratio is a clinically relevant marker of surgical stress in patients with colorectal cancer. Cytokine 2011;53:243-8. [Crossref] [PubMed]
- Gogos CA, Drosou E, Bassaris HP, et al. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 2000;181:176-80. [Crossref] [PubMed]
- Ugras B, Giris M, Erbil Y, et al. Early prediction of anastomotic leakage after colorectal surgery by measuring peritoneal cytokines: prospective study. Int J Surg 2008;6:28-35. [Crossref] [PubMed]
- Leng SX, McElhaney JE, Walston JD, et al. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci 2008;63:879-84. [Crossref] [PubMed]


