
Clinical outcomes with first-line chemotherapy versus endocrine therapy for adjuvant endocrine therapy-resistant metastatic breast cancer
Introduction
Breast cancer has the highest incidence and is second in mortality rate of any cancer among women (1). A high percentage of breast cancers, 50–70%, are hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) (2,3). The main cause of death from breast cancer is metastasis, which develops in 20–30% of patients with early-stage breast cancer and in 6–10% of newly diagnosed breast cancer cases (4,5).
Metastatic breast cancer (MBC) is not a curable disease; therefore, the goal is to prolong survival and maintain patient quality of life. Typically, there are two treatment options for HR+/HER2− MBC patients, endocrine therapy (ET) and chemotherapy (CT) (6). The recommendation for treating patients with ET as first-line therapy was essentially based on a previous study (7). Results from that study showed that there was no significant difference in overall survival (OS) between ET- and CT-treated patients. CT was associated with an increased response rate and toxicity. Therefore, it is recommended that ET be used before CT, except in patients with rapidly progressive disease.
ET resistance (ETR) is a great obstacle in the treatment of HR+/HER2− MBC. Although patients are encouraged to receive three consecutive cycles of ET treatment (8), the clinical benefit rate (CBR) declines rapidly from 70–30% or even lower (9,10). Thus, additional evidence is required in choosing CT or ET in clinical practice.
The purpose of this study was to explore the factors that affect the clinical outcomes of ET and CT in the first-line treatment of patients with ETR HR+/HER2− MBC.
Methods
Patients
In total, 405 consecutive patients with ER+/HER2− MBC treated in the Department of Breast Oncology of Beijing Cancer Hospital (Beijing, China) between June 2013 and June 2015 were retrospectively analyzed. Of these, 135 patients were selected with adjuvant ETR according to the following inclusion criteria: female MBC patients aged ≥18 and ≤75 years with ER+/HER2− primary breast cancer who were administered ET or CT as their first-line treatment. ETR was defined as relapse during adjuvant ET or within 12 months after completing adjuvant ET.
Demographic and clinicopathological data were recorded from electronic medical records. The subsequent maintenance ET followed by first-line CT and the second-line therapy regimen were also recorded. The ethics committee of Beijing Cancer Hospital (Beijing, China) approved this study and written informed consent was obtained from all patients.
ER- and/or progesterone receptor (PR)-positivity was defined as immunohistochemical staining of more than 1% of cells according to current guidelines (11). ER staining of more than 10% of cells was defined as high. Ki-67 index high was defined as >20% positive cells. HER2-negativity was defined as tumors with a HER2 immunohistochemical score of 0 or 1+, or 2+ and FISH negative.
Clinical outcome definitions
RECIST criteria 1.1 was used to assess treatment outcomes (12). The objective response rate (ORR) was defined as the proportion of patients with a complete or partial response (CR + PR/ALL). The CBR was defined as the proportion of patients with a complete or partial response or with stable disease at week 24 (CR + PR + SD ≥24 weeks/ALL). Progression-free survival (PFS) was defined as the interval between commencement of therapy and tumor progression or death. OS was defined as the interval between the commencement of first-line treatment and death from any cause.
Statistical analysis
Demographic and clinicopathological characteristics of patients were grouped as continuous variables and categorical variables. Continuous variables were presented as the median and range. Categorical variables were described as frequency. The baseline characteristics of patients and the response rate between ET and CT were compared using Pearson’s χ2 test or Fisher’s exact test. Univariate and multivariate logistic regression analysis were used to explore the factors affecting the ORR of ET vs. CT. The Kaplan-Meier method was used to calculate the PFS of ET and CT and the log-rank test was used to compare the PFS of ET and CT. Multivariable Cox proportional hazards regression analysis was used to examine the association of potential influential factors with PFS in first-line treatment. P≤0.05 was considered statistically significant. The statistical package for the social sciences (SPSS) software version 18.0 was used for the analysis.
Results
Patient characteristics
There were 96 (71.1%) patients that received CT and 39 (28.9%) that received ET as their first-line treatment in the study. Baseline patient characteristics are shown in Table 1. Patients in both ET and CT groups had similar clinicopathological characteristics. More patients in the first-line ET group had tumors with invasive lobular cancer and grade I tumors than those in the first-line CT group (P=0.007, P=0.001). However, patients receiving first-line CT had significantly higher frequent visceral metastasis (P=0.001). Among the 39 patients receiving ET, 26 (66.7%) received an aromatase inhibitor (AI), 6 (15.4%) received tamoxifen or toremifene, 3 (7.7%) received fulvestrant, 2 (5.1%) received goserelin and an AI, 1 received only goserelin, and 1 (2.6%) received everolimus and an AI. The most common agent administered to those in the CT group was a taxane (79.2%), followed by capecitabine (43.8%), gemcitabine (36.5%), anthracycline (8.3%), and vinorelbine (6.3%).
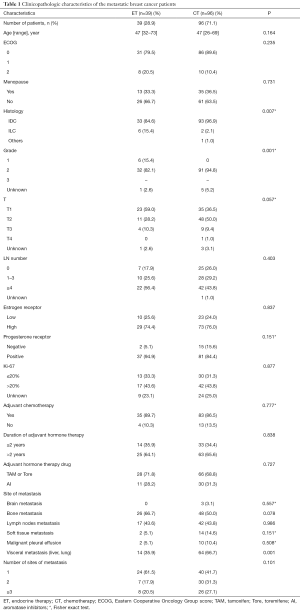
Full table
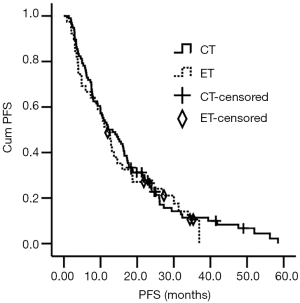
Median follow-up was 41.0 months. The ORR to ET was 2.6%, which was significantly lower than that to CT (37.5%) (P<0.001). The median PFS was 12.0 (95% CI, 9.1–15.0) months for the whole population. The median PFS in the ET and CT groups was 11.8 (95% CI, 8.3–15.3) months and 12.0 (95% CI, 7.6–16.4) months, respectively, which was not significantly different (P=0.667) (Figure 1, Table 2). There were nine deaths until the last follow-up. The median OS of the nine patients was 39.5 (8.4–62.7) months. Therefore, we did not compare OS between the two groups.
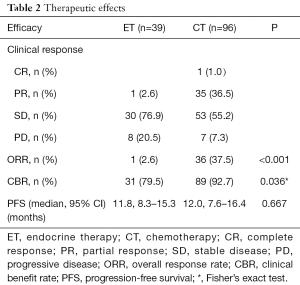
Full table
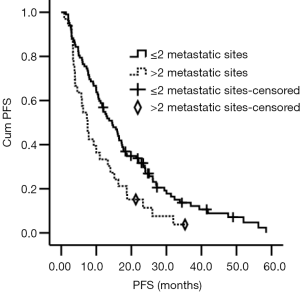
To explore factors that affected the ORR, we combined all potential factors into a single factor and used multivariate logistic regression analysis. It showed that bone metastasis and first-line treatment (ET or CT) had significantly affected ORR (14.9% vs. 42.6%, P=0.008, OR =0.185; 37.5% vs. 2.6%, P=0.025, OR =0.079). In multivariate Cox regression analysis, patients with more than two metastatic sites had a shorter PFS than patients with less than or equal to two metastatic sites, which amounted to 7.5 and 14.5 months, respectively (P=0.031, HR =1.714) (Figure 2, Table 3).
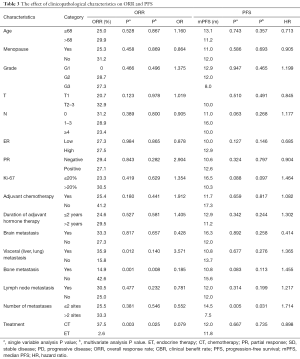
Full table
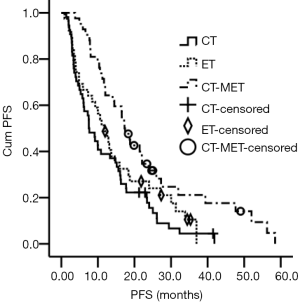
Then patients with CT were further stratified according to maintenance ET (MET) after CT. Patients on MET had significantly longer PFS (14.3, 95% CI, 14.9–19.7) months than those not on MET (7.5, 95% CI, 5.2–9.9) months, or those on ET as first-line therapy (11.8, 95% CI, 8.3–15.3) months (P=0.003) (Figure 3).
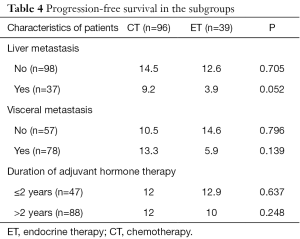
Patients receiving CT had more frequent liver and visceral metastasis. In addition, the duration of adjuvant ET less than 2 years indicates primary ET resistance. Therefore, we further analyzed PFS in ET and CT subgroups of liver, visceral metastasis, and duration of adjuvant ET of less or more than 2 years. We found no significant difference of PFS in any of these ET and CT subgroups (Table 4).
Full table
Discussion
In our study, more than two-thirds of patients with adjuvant ETR received CT in real-world clinical practice. ET is recommended for the patients with ER+/HER2− breast cancer except in cases of rapidly progressive, symptomatic disease, or visceral metastasis at risk of end-organ dysfunction (often termed visceral crisis) (13). Patients with ETR had a greatly decreased CBR with ET, from approximately 70% with first-line therapy to 30% for following lines of therapy. Therefore, ETR is one of the clinical concerns that impacts therapeutic choice. Our finding was also confirmed in a previous report of first-line treatment for patients with MBC in China, in which they showed that HR+ patients who received first-line CT accounted for 97.7% (589/603), while only 1% of patients received ET. That is the reality in China, where a large proportion of ER+/HER2− patients receive CT as first-line treatment.
In our study, more patients with invasive ductal carcinoma, higher histologic grade, liver or visceral metastasis received CT. All these factors were associated with a poor prognosis. However, we found that PFS was not significantly different between patients administered ET and those who received CT (P=0.667). The above factors did not significantly affect the ORR or PFS. The main international guidelines recommend ET as first-line treatment in HR+/HER2− MBC (14,15), which has the same survival benefit with less toxicity and better quality of life compared with CT (14-16). CT is not recommended as first-line therapy for ER+/HER2− breast cancer, even in patients with adjuvant ETR.
Most patients with ET in our study received AIs, which are effective in patients with tamoxifen-resistant MBC as second-line treatment in postmenopausal MBC patients (17). AIs decrease estrogen levels by inhibiting biosynthesis of estrogen (18), which is different from an estrogen receptor modulator (19). In our study, most of the patients who were administered ET (71.8%) received tamoxifen or toremifene in the adjuvant setting. This partly explained the clinical benefit in patients with ETR. Therefore, it is recommended to consider second- and third-line ET for ER+/HER2− patients who have no urgent need for CT.
Patients who received MET in the CT group had the longest median PFS. Maintenance therapy is recommended for patients who benefit from first-line treatment according to current guidelines. Previous trials have shown that maintenance CT extended the duration of remission (20-23), but improvement in OS was not observed in most trials (24). Nevertheless, toxicity was significantly increased in the maintenance CT arm in most studies. Therefore, MET is a commonly employed strategy aimed at decreasing treatment-related adverse events, without compromising OS in the treatment of ER+/HER2− MBC patients (25).
A multicenter retrospective study involving 314 HER2− MBC patients and 12 cancer centers evaluated the impact of paclitaxel-bevacizumab, maintenance therapy with bevacizumab (BM), and ET in real-world practice. The result from the study confirmed that MET significantly improved PFS and OS compared with no maintenance therapies (26). In another study, bevacizumab with or without hormone therapy was used as maintenance therapy after first-line paclitaxel plus bevacizumab in patients with HER2− HR+ MBC. Median PFS in patients who received maintenance bevacizumab with hormone therapy was longer than in those who did not receive hormone therapy (13 and 4.1 months, respectively, P=0.05). Maintenance bevacizumab was also found to be well tolerated (27). Dufresne et al. (28) reported that patients benefitted from MET when it was given after first-line CT. MET significantly prolonged PFS from 7.8 to 16.3 months (P<0.0001). There have been two prospective trials exploring the effect of MET (29,30). In one study, patients who received medroxyprogesterone acetate (MPA) as maintenance therapy had longer median time to progression (TTP) (4.9 vs. 3.7 months; P=0.02) compared with the control group. However, there was no difference in OS (17.4 vs. 18.3 months; P=0.39) between the two groups. In another letrozole-based single-arm phase II study, the median TTP was as long as 18.5 months, and 15.5% of patients had a better response status during letrozole treatment, which was well tolerated and did not significantly affect quality of life. Although these two previous studies suggest a clinical benefit of MET, they each have their own limitations: small sample size, rarely used drug (MPA) in current practice, and a lack of a control arm in the letrozole study. Trédan et al. (31) also reported that ER+, HER2− MBC patients with no evidence of progression after first-line taxane + bevacizumab did not achieve longer PFS with maintenance therapy with exemestane + bevacizumab compared with continuation of taxane + bevacizumab in a phase III trial. With these divergent results, it would be too early to draw conclusions regarding the usefulness of MET. Nevertheless, given that MET is well tolerated and improves survival, it might indicate that this treatment strategy should be appropriate for ER+/HER2− MBC patients.
This study had several limitations. First, it was a retrospective analysis in which the baseline between groups was not well balanced. More cases with severe conditions were treated with CT. Second, the baseline quality of life of patients was recorded as an Eastern Cooperative Oncology Group (ECOG) score. However, it was insufficient to reflect the physicians’ consideration while making the treatment choice.
In real-world practice, ET and CT are both appropriate treatments for patients with ETR. MET is a good choice for ER+/HER2− patients. Prospective studies are warranted to further compare ET and CT and explore the sequence of these treatments in ETR MBC.
Acknowledgments
We thank Mark Abramovitz, PhD, from Liwen Bianji, Edanz Group China (
Funding: General Project of Beijing Natural Science Foundation “Detection and molecular phenotyping of circulating tumor cells in breast cancer based on specific recognition peptide” (item number: 2162044 to Yanlian Yang, execution time: 2016.7–2018.12).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.43). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics committee of Peking University Cancer Hospital (2016YJZ33). Written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010;28:3271-7. [Crossref] [PubMed]
- Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014;106: [Crossref] [PubMed]
- Lu J, Steeg PS, Price JE, et al. Breast cancer metastasis: challenges and opportunities. Cancer Res 2009;69:4951-3. [Crossref] [PubMed]
- O'Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist 2005;10:20-9. [Crossref] [PubMed]
- Chung CT, Carlson RW. Goals and objectives in the management of metastatic breast cancer. Oncologist 2003;8:514-20. [Crossref] [PubMed]
- Wilcken N, Hornbuckle J, Ghersi D. Chemotherapy alone versus endocrine therapy alone for metastatic breast cancer. Cochrane Database Syst Rev 2003;CD002747 [PubMed]
- Gradishar WJ, Anderson BO, Balassanian R, et al. Invasive Breast Cancer Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:324-54. [Crossref] [PubMed]
- Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol 2008;26:1664-70. [Crossref] [PubMed]
- Ellis MJ, Llombart-Cussac A, Feltl D, et al. Fulvestrant 500 mg Versus Anastrozole 1 mg for the First-Line Treatment of Advanced Breast Cancer: Overall Survival Analysis From the Phase II FIRST Study. J Clin Oncol 2015;33:3781-7. [Crossref] [PubMed]
- Goldhirsch A, Wood WC, Gelber RD, et al. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol 2003;21:3357-65. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Parnes HL, Cirrincione C, Aisner J, et al. Phase III study of cyclophosphamide, doxorubicin, and fluorouracil (CAF) plus leucovorin versus CAF for metastatic breast cancer: Cancer and Leukemia Group B 9140. J Clin Oncol 2003;21:1819-24. [Crossref] [PubMed]
- Partridge AH, Rumble RB, Carey LA, et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2014;32:3307-29. [Crossref] [PubMed]
- Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast 2014;23:489-502. [Crossref] [PubMed]
- Higgins MJ, Wolff AC. Therapeutic options in the management of metastatic breast cancer. Oncology (Williston Park) 2008;22:614-23; discussion 623, 27-9.
- Bliss JM, Kilburn LS, Coleman RE, et al. Disease-related outcomes with long-term follow-up: an updated analysis of the intergroup exemestane study. J Clin Oncol 2012;30:709-17. [Crossref] [PubMed]
- Miller WR. Aromatase inhibitors: mechanism of action and role in the treatment of breast cancer. Semin Oncol 2003;30:3-11. [Crossref] [PubMed]
- Geisler J. Differences between the non-steroidal aromatase inhibitors anastrozole and letrozole--of clinical importance? Br J Cancer 2011;104:1059-66. [Crossref] [PubMed]
- Muss HB, Case LD, Richards F 2nd, et al. Interrupted versus continuous chemotherapy in patients with metastatic breast cancer. The Piedmont Oncology Association. N Engl J Med 1991;325:1342-8. [Crossref] [PubMed]
- Gennari A, Stockler M, Puntoni M, et al. Duration of chemotherapy for metastatic breast cancer: a systematic review and meta-analysis of randomized clinical trials. J Clin Oncol 2011;29:2144-9. [Crossref] [PubMed]
- Alba E, Martin M, Ramos M, et al. Multicenter randomized trial comparing sequential with concomitant administration of doxorubicin and docetaxel as first-line treatment of metastatic breast cancer: a Spanish Breast Cancer Research Group (GEICAM-9903) phase III study. J Clin Oncol 2004;22:2587-93. [Crossref] [PubMed]
- Beslija S, Bonneterre J, Burstein H, et al. Second consensus on medical treatment of metastatic breast cancer. Ann Oncol 2007;18:215-25. [Crossref] [PubMed]
- Park YH, Jung KH, Im SA, et al. Phase III, multicenter, randomized trial of maintenance chemotherapy versus observation in patients with metastatic breast cancer after achieving disease control with six cycles of gemcitabine plus paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol 2013;31:1732-9. [Crossref] [PubMed]
- Stockler M, Wilcken NR, Ghersi D, et al. Systematic reviews of chemotherapy and endocrine therapy in metastatic breast cancer. Cancer Treat Rev 2000;26:151-68. [Crossref] [PubMed]
- Gamucci T, Mentuccia L, Natoli C, et al. A Real-World Multicentre Retrospective Study of Paclitaxel-Bevacizumab and Maintenance Therapy as First-Line for HER2-Negative Metastatic Breast Cancer. J Cell Physiol 2017;232:1571-8. [Crossref] [PubMed]
- Fabi A, Russillo M, Ferretti G, et al. Maintenance bevacizumab beyond first-line paclitaxel plus bevacizumab in patients with Her2-negative hormone receptor-positive metastatic breast cancer: efficacy in combination with hormonal therapy. BMC Cancer 2012;12:482. [Crossref] [PubMed]
- Dufresne A, Pivot X, Tournigand C, et al. Maintenance hormonal treatment improves progression free survival after a first line chemotherapy in patients with metastatic breast cancer. Int J Med Sci 2008;5:100-5. [Crossref] [PubMed]
- Kloke O, Klaassen U, Oberhoff C, et al. Maintenance treatment with medroxyprogesterone acetate in patients with advanced breast cancer responding to chemotherapy: results of a randomized trial. Essen Breast Cancer Study Group. Breast Cancer Res Treat 1999;55:51-9. [Crossref] [PubMed]
- Bertelli G, Garrone O, Bertolotti L, et al. Maintenance hormone therapy with letrozole after first-line chemotherapy for advanced breast cancer. Oncology 2005;68:364-70. [Crossref] [PubMed]
- Trédan O, Follana P, Moullet I, et al. A phase III trial of exemestane plus bevacizumab maintenance therapy in patients with metastatic breast cancer after first-line taxane and bevacizumab: a GINECO group study. Ann Oncol 2016;27:1020-9. [Crossref] [PubMed]


